blog
March 11, 2014 | Source: Rapid Micro Biosystems, Inc.
Accelerating Biological Indicator Testing with The Growth Direct™ System
POSTED BY Rapid Micro Biosystems | 4 minute read
March 11, 2014 | Source: Rapid Micro Biosystems, Inc.
POSTED BY Rapid Micro Biosystems | 4 minute read
 With the aid of growth-based rapid microbiological methods (RMM) technologies, pharmaceutical quality control departments are able to speed up their bioburden, environmental monitoring and sterility testing, while also achieving greater accuracy and reliability compared to the compendial methods. Through the application of sophisticated imaging equipment, automated sample handling and immediate reporting, microbiologists can now perform high-volume testing while at the same time reducing labor and improving time to results.
With the aid of growth-based rapid microbiological methods (RMM) technologies, pharmaceutical quality control departments are able to speed up their bioburden, environmental monitoring and sterility testing, while also achieving greater accuracy and reliability compared to the compendial methods. Through the application of sophisticated imaging equipment, automated sample handling and immediate reporting, microbiologists can now perform high-volume testing while at the same time reducing labor and improving time to results.
Additionally, RMM technologies can streamline applications beyond environmental monitoring, sterility and bioburden testing. Take biological indicator (BI) testing, for instance, a critical step in the qualification of sterilization processes. Biological indicators are standardized preparations of microorganisms with known resistances to sterilization processes such as steam, dry heat and radiation. The basic BI types include:
BIs are typically tested in two ways, and each test takes 2-7 days when using a compendial method. First, manufacturers test new BI lots upon reception to ensure they contain the appropriate species and numbers of spores specified. Also, testing is performed to establish pass\fail criteria for use in process or validation testing. The established pass\fail criteria are then used for release testing. Of course, any product, equipment or packing materials will be held in quarantine until the results are available.
This process can be sped up by implementing The Growth DirectTM System, which can detect spore microcolonies days before they grow large enough to become visible to the naked eye. Users can achieve reliable results in roughly half the time of standard compendial methods. The graph below illustrates the system's time to results for four different BI preparations – less than 24 hours in each case – which would normally require three days of incubation or more.
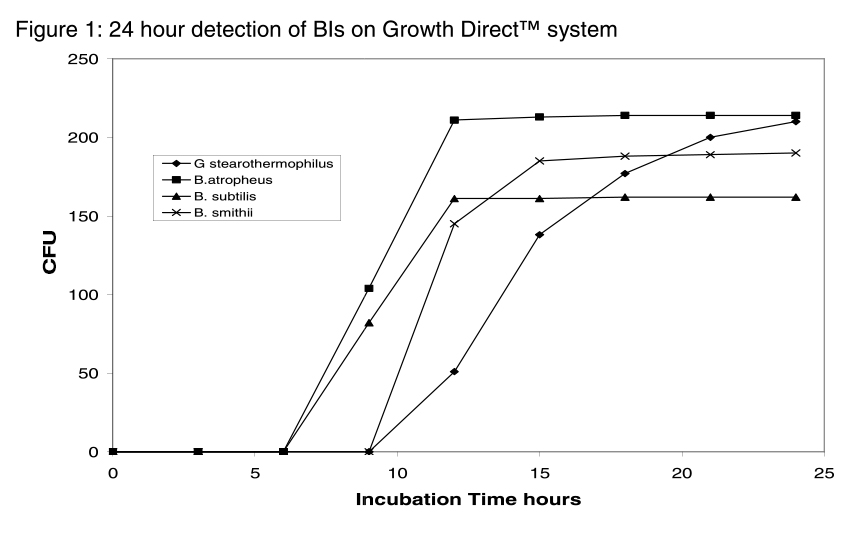
The Growth DirectTM System's high detection range of 1500 CFU means that fewer dilutions of the test suspension are required. BI concentrations can be as high as 108 CFU / mL, and since no more than 250 colonies can be detected accurately by visual counting methods, often multiple, repeated dilutions may be required.. Each of these dilutions costs additional time and materials and presents an additional opportunity for human error.
The figures below compare the accuracy of The Growth DirectTM System to traditional Petri plate counts with different species used for BIs and also with a stressed preparation of B. subtilis to mimic the a spore population that was exposed to a sterilization process. In both cases the Growth DirectTM System exhibited equivalent detection of colonies in 24 hours as were visually counted on the control spread Petri plates after 3 days incubation.
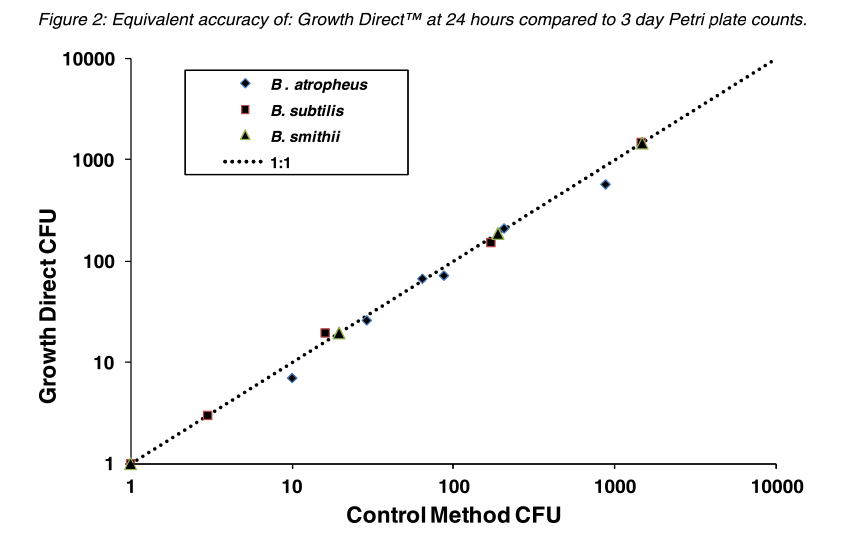
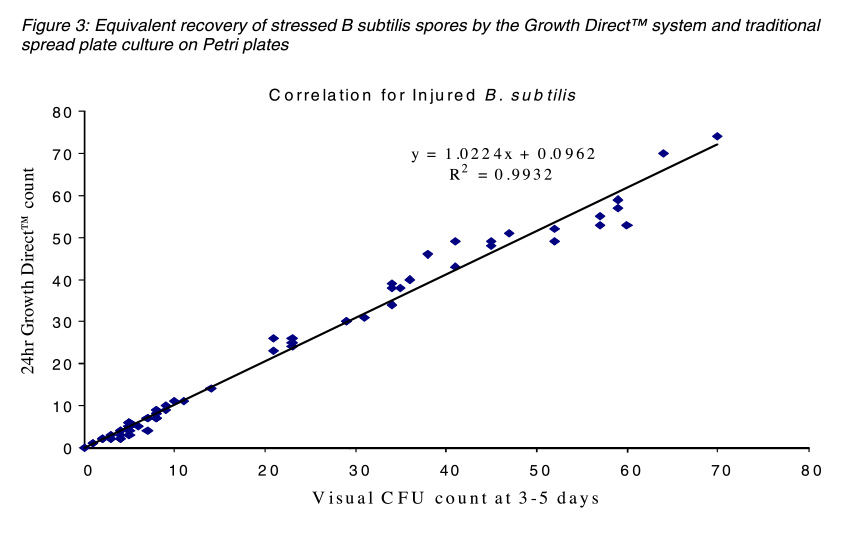
Overall, testing has shown that the Growth DirectTM System can detect the same numbers of colonies in 24 hours as detected using the traditional three-day incubation method. In addition, system automation leads to lower resource requirements.
Learn more about how The Growth Direct™ System affected bioburden testing in a model sample matrix for influenza by simply clicking here.