blog
April 30, 2013 | Source: Admin User, Inc.
Automated Rapid Detection in a Model Sample Matrix for Flu Vaccine
POSTED BY Admin User | 5 minute read
April 30, 2013 | Source: Admin User, Inc.
POSTED BY Admin User | 5 minute read
Customers often come to use with "unique" products for testing using the Growth Direct™ technology. Part of role of the Rapid Micro Biosystems Technical Services team is to perform feasibility studies on certain types of samples to determine how it would be tested on the Growth Direct System and what rapid detection results are acheived. The team has a well-defined set of processes to test samples and present the information in the form of an application note.
As I mentioned above, these studies are sometimes driven by our customers, but we regularly perform our own internal feasibility studies to test the limits of the Growth Direct System and our rapid retection technology.
Below is a study focused on a model matrix for the influenza virus. The study is also in the rapid library.
Background:
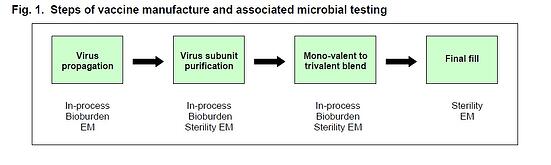
The manufacture of influenza vaccine has four major steps: virus propagation, harvest and processing, blending of bulk stocks, and final fill of the finished vaccine (Fig. 1). Influenza virus is traditionally grown using chicken eggs inoculated with seed virus, though cell culture-based methods are now becoming established. Whether egg- or cell culture-based methods are used, propagated virus is then harvested and processed to extract and purify the specific viral subunits required to produce mono-valent bulk stock for vaccine. These stocks are subsequently blended into trivalent bulk which is loaded into the final packaging for release.
Throughout the manufacturing process an assortment of microbial QC assays must be performed to confirm that microbial contaminants are maintained within appropriate limits (e.g. purification steps), or have been completely removed (e.g. final fill). Fig. 1 lists the types of testing performed during each major step of vaccine manufacture. Additionally, environmental monitoring (EM) consisting of air, surface and personnel testing is carried out throughout production to confirm that the manufacturing process is performed under controlled conditions. Microbial QC testing requires from 3 to 14 days to complete, is performed at multiple production steps, and progression to each subsequent step may be contingent upon passing these lengthy tests (e.g. 14 days for sterility); consequently such testing can have significant additive impacts on the production cycle. These procedures also require substantial resources (i.e. labor and materials) to perform. Thus, reducing the turn-around times for microbial QC results could have a significant impact on influenza vaccine production cycle time, costs, and the timing of product distribution to the market.
Application:
The Growth Direct™ System for rapid microbial enumeration addresses all the applications required for microbial QC testing in vaccine manufacture. It uses the same principles and methods as the compendia, thus avoiding the extensive validation work often found with other rapid testing platforms, while fully automating incubation, sample handling, analysis, and results reporting.
Only sample preparation is performed by the user (i.e. standard membrane filtration method). Finally, it is a non-destructive test; this attribute allows subsequent microbial ID, a necessity for root cause investigation and contamination prevention.
Conclusion:
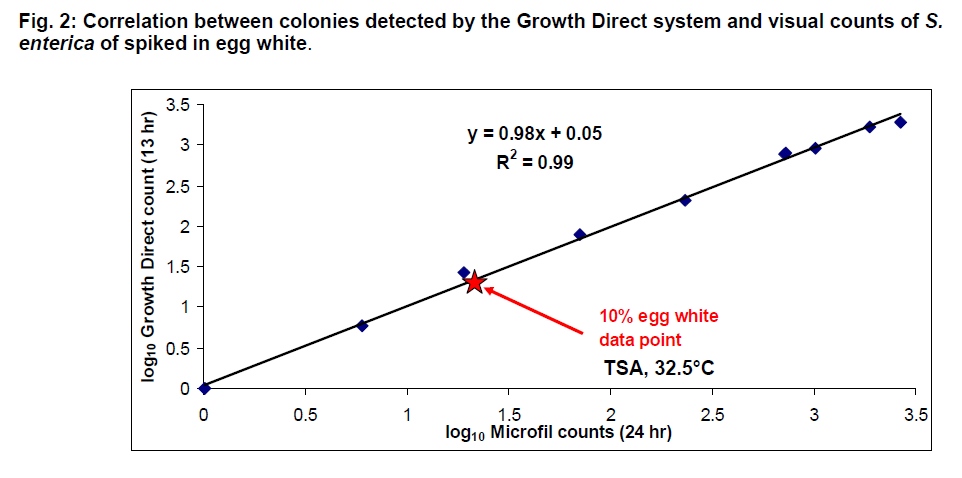
This study demonstrates that microbial contaminants in egg white, a model matrix for allantoic fluid, can be rapidly analyzed on the Growth Direct system. Equivalent numbers of colonies were detected by the Growth Direct in 13 hours as were counted via the culture method after 24 hrs: a 50% time savings. Such time savings combined with lower labor costs and improved compliance from system automation can:
These benefits can result in a payback on the initial investment of the Growth Direct system of two years or less, due to the resulting substantial improvements in efficiency and productivity, and cost savings realized by the vaccine manufacturer.