blog
March 27, 2017 | Source: Admin User, Inc.
How to increase efficiency and savings with a single incubation strategy for environmental monitoring
POSTED BY Admin User | 5 minute read
March 27, 2017 | Source: Admin User, Inc.
POSTED BY Admin User | 5 minute read
Today, the main goal of rapid microbiology methods (RMMs) for environmental monitoring (EM) is to reduce the incubation times of standard serial methods and achieve a faster time to result (TTR).
The faster TTR of the RMM gives us the data we need more quickly so we can take the proper actions on the sample, whether it be product release or confirmation that critical processes are operating within microbiological specification.
An important component for implementing RMM is choosing the appropriate TTR for a testing application. But this choice demands a balance between speed and accuracy of test results.
An excessively long TTR may reduce any advantages of an RMM, and can cast doubt on the rationale for its use, especially if saving time is the primary goal. A TTR that is too short can lead to an inaccurate result – contamination isn’t detected, for instance – thus endangering the process or product.
Now, labs can achieve both increased lab efficiency and significant savings in EM by replacing a standard serial incubation test with a single temperature incubation strategy.
A recent article published in the European Journal of Parenteral & Pharmaceutical Sciences describes a method to determine incubation regime and TTR for automated RMM for environmental monitoring, including the use of a single incubation temperature to replace a standard serial incubation profile.1
The incubation temperature chosen was 28°C, the mid-point between 32.5°C and 22.5°C. This temperature has been investigated as an alternative to serial incubation because it is thought to be low enough to allow the recovery of temperature-sensitive species that would not grow at 32.5°C, while the TTR determined would remain comparable to that obtained for serial incubation.
The species used were temperature-sensitive molds Penicillium brevicompactum and Cladosporium halotolerans. Each was tested by the Growth Direct™ System at 28°C for 5 days.
Over the course of the incubation, counts were detected and the percentage of CFU was plotted for each time-point versus the final count (Figure 1). The temperature-sensitive molds grew at this temperature successfully, as did the other organisms.
Figure 1. Use of 280C reduces TTR while allowing recovery of all strains.
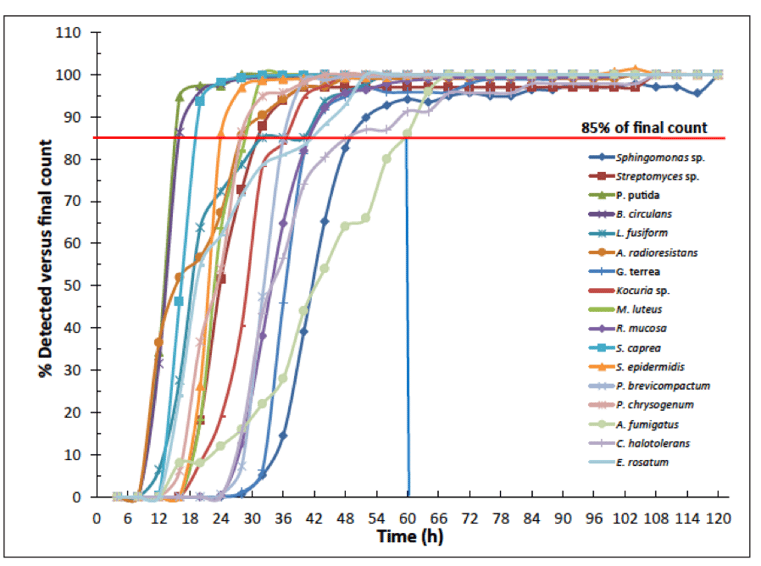
Based on the detection plot, the TTR for 28°C was determined to be 60 h for the organisms tested. This was lower than that determined for the serial incubation TTR of 72 h also described in the article.
In addition, recovery of the isolates at the determined 60 h TTR was ≥ 70% of the spread controls for these organisms (Table 1)
Table 1. Comparable recovery of test isolates at 60 h280C incubation versus 5-day spread plate control. 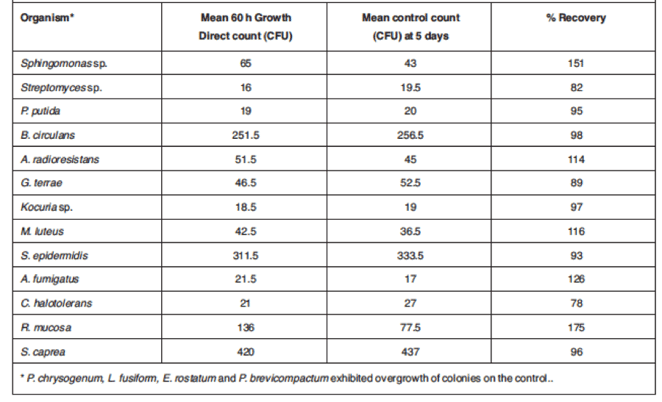
Based on the findings of the article, replacing a standard serial incubation profile with a single temperature incubation strategy for EM helps labs achieve the delicate balance of fast, accurate test results—and reach their goals of increasing efficiency and cost savings.
For more information on a single temperature incubation strategy for EM and to learn how automated compendial testing can help empower your manufacturing facility or lab with the power of automation, contact Rapid Micro Biosystems.
References
1. Sage A, Timas N, Jones D. Determining incubation regime and time to results for automated rapid microbiology EM methods. European Journal of Parenteral & Pharmaceutical Sciences. 2014;19(2):45-55.