blog
May 5, 2020 | Source: Rapid Micro Biosystems, Inc.
[Podcast] Maintaining Operational Efficiencies in QC Micro Labs During a Pandemic
POSTED BY Rapid Micro Biosystems | 6 minute read
May 5, 2020 | Source: Rapid Micro Biosystems, Inc.
POSTED BY Rapid Micro Biosystems | 6 minute read
COVID-19 makes this one of the more interesting times to introduce our new podcast, which is why we decided to focus it on a critical topic: How to maintain operational efficiencies in your QC lab during an unprecedented time.
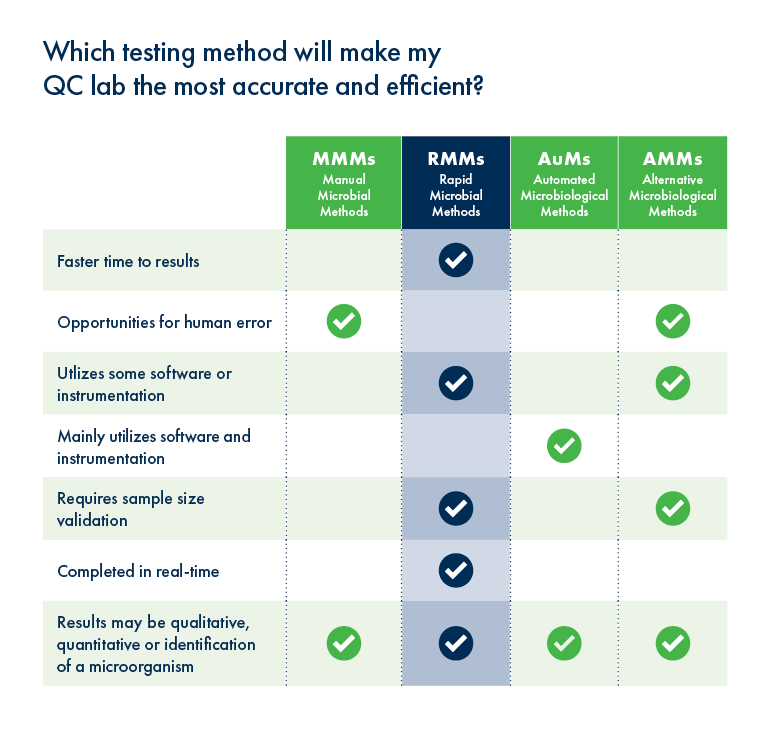
We wondered how the workflow of Growth Direct is helping QC teams manage their pandemic response.
Both Susan and David agreed that the ability to run a virtually hands-free operation was a huge benefit as social distancing continues to be critical for worker safety. Growth Direct makes this possible because once the samples are taken, all of the other steps in the process are automated. David also pointed out that Growth Direct has a remote monitoring system, so technicians or engineers no longer need to be onsite to troubleshoot or manipulate the machine. He added that remote monitoring on an international scale is not just possible but currently necessary for some systems, and RMB has been innovative enough to ensure that those activities continue without interruption.
If your QC micro lab is considering new operational efficiencies, we hope this podcast conversation helped you better understand the benefits of automation.
Listen to the full episode above and be sure to subscribe through the form top right to be the first to receive future episodes.
Looking to learn more?
The impact of COVID-19 has been felt by everyone. Those business teams that are learning from experience, taking time to consider and implement new processes and innovations stand to benefit the most.
Related Articles:
QC Labs That Employ Rapid Microbiological Methods Are Better Positioned to Endure the COVID-19 Pandemic
About Our Guests
Susan Schilling
Director of Customer Relations
sschilling@rapidmicro.com
Susan is an expert in rapid microbiology methods, and she has focused her entire sales career on bringing new concepts for improved efficiency, faster results, and superior quality to pharmaceutical quality control laboratories.
She has been with RMB for nearly ten years, partnering with pharmaceutical customers in the area of microbiology innovation to determine the best solution for their facility. Previously, Susan spent over 19 years at bioMerieux as Regional Business Manager for the eastern region of biopharma industry sales team.
David Jones, Ph.D.,
Director of Technical Marketing and Industry Affairs
djones@rapidmicro.com
Dr. Jones has more than 20 years of experience with rapid microbial detection technology, laboratory instrumentation development, and laboratory management, both European and U.S. markets.
Previously, David held positions with Wyeth Biopharmaceutical as Manager of Microbial Test Technologies. He was responsible for the introduction and validation of new technologies to improve microbiology and molecular biology QC quality and efficiency.
Prior to Wyeth, David held several positions at Chemunex in France, including acting as Project Manager for the ScanRDI® rapid microbial detection systems. As Director of QA and Regulatory Affairs, David headed up the QC function and led the successful customer implementation and validation of the Scan RDI for water and in-process testing in Europe and the United States. In this role, David worked closely with the FDA and USP organizations.
David has authored a number of papers on the validation of new technologies. He is also on the TR33 committee updating the guidelines to Pharma for validation of Rapid Microbiology