blog
November 18, 2020 | Source: Rapid Micro Biosystems, Inc.
PDA Micro Insights from the Contamination Control Strategy Technical Report
POSTED BY Rapid Micro Biosystems | 9 minute read
November 18, 2020 | Source: Rapid Micro Biosystems, Inc.
POSTED BY Rapid Micro Biosystems | 9 minute read
Last month PDA gathered a panel of thought leaders in the contamination control space together to discuss their work on the upcoming Contamination Control Technical Report (TR), which will take a more holistic approach to contamination control strategy (CCS) fundamentals and best practices. This team of experts have been working on this document for nearly two years, and it is anticipated to be published in early 2021.
The panel, which took place at the 15th Annual PDA Global Conference on Pharmaceutical Microbiology, was moderated by Christine Sherman, Sterilization and Aseptic Processing leader at Takeda, and included Cheryl Essex, Contamination Control expert at Sanofi, Dr. Ed Tidswell, Executive Director of the Sterile and Microbiology QA Organization at Merck, and Jeanne Mateffy, Chief Microbiologist/Director of Quality Sciences and Global Process Owner of Contamination Control at Amgen.
Here are some highlights from the Contamination Control Strategy Technical Report session.
The panel was asked, “Contamination control strategies (CCSs) have been around for a while now, how does the content of this TR distinguish itself? What new aspects, principals, and concepts does TR bring to the table?”
The answer was that there are four distinguishing factors for this technical report:
While elements of this guidance document have been presented on and written about previously, it is the holistic approach and the focus on the fundamentals that will make this report so valuable to the pharmaceutical industry.
All members of the panel emphasized that this is a technical report they wish it had been available when they were starting out. Cheryl Essex referenced the house analogy that she presented at the 2018 ISPE Quality Manufacturing conference: If visualizing CCS, the foundational elements are knowledge; quality culture; and quality risk management. Built on that foundation are pillars, including how you control your facility; raw materials; process; and personnel. Quality risk management helps you to assess how each of those pillars will be built, but there is no “one” risk assessment to rule them all. It’s simply a way of thinking about how to craft the strategy. This approach is integral to helping build a holistic contamination control strategy from the ground up.
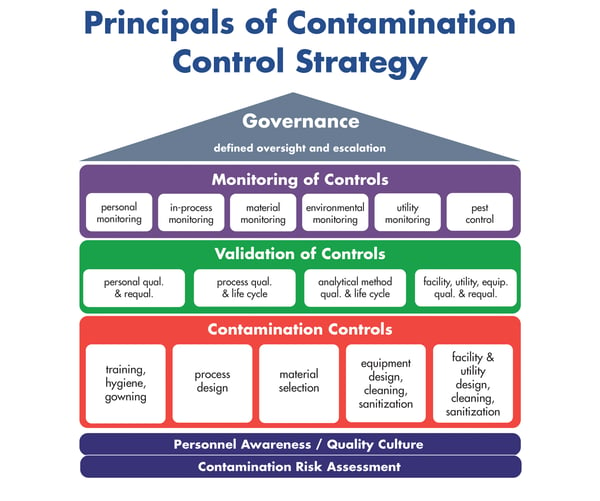 Later in the discussion, an audience member asked, “Can you expand on human factors within CCS framework?”
Later in the discussion, an audience member asked, “Can you expand on human factors within CCS framework?”
In the Technical Report there is a section on “Foundational Elements of CCS.” Within that section, there are human factors listed that include: scientific knowledge; quality risk management; quality culture; and personal awareness. This section dives into factors that have to be in place for the foundational elements to be strong. For instance, it will affect every other control in the control strategy if the foundation is not solid. In this section, there is also guidance on roles and responsibilities and how to remove individual human biases and allow diverse thinking.
One of the key questions the panelists were asked during the discussion was, “Which department should take ownership of the CCS?”
Overall, the panel agreed that an organization cannot be successful at implementing the CCS without cross-functional inclusion at all levels, including microbiologists, technologists, manufacturing, technical operations, and new product teams. However, there needs to be a leader who takes ownership of the contamination control strategy and works horizontally to include other key groups. Cheryl Essex noted at Sanofi the leader is Contamination Control or Sterility Assurance group, it is practical for them to manage and update the documentation on a regular basis while including input from other departments. Jeanne Mateffy added the Process Development group often kicks off the contamination control strategy discussion, but the manufacturing site will ultimately own the process with Quality Assurance and is involved at every step.
The follow-up was then asked, “Should sites separate the product CCS from the site CCS?”
The panel replied no, the strategy needs to be holistic and that “everything we do is to keep the product uncontaminated,” but if you are looking for commonalities and ways to share best practices then you may be able to group the strategies and controls for like manufacturing processes.
The team was also asked, “How often should the site review the CCS document?”
The panel agreed that CCS should be regularly reviewed to ensure best practices are followed. Moderator, Christine Sherman, pointed out that because the CCS is a living document, it must be updated as the manufacturing situation evolves. But the question remains: what triggers those changes? Cheryl Essex says, “All change controls that touch upon any contamination control element need to have an evaluation and could trigger an update to the CCS.” In addition, panel participants agreed that a periodic review is necessary to ensure the CCS is up to date. The panel suggested that it would be necessary to do so on an annual basis even if there are triggers at other times that require changes to the TR. If microbiology teams are looking to drive changes and updates to the contamination control strategy, they should look to partner with the new products team, as the importance of new products can help bring visibility to needed updates.
Before implementing or expanding your own contamination control strategy, consider assessing your current QC Micro methods and the ways you may be putting your organization at unnecessary risk.
Download and complete our QC Micro Performance Questionnaire to get started today or contact us directly for help identifying gaps in your existing controls.
Also, don’t miss out the opportunity to visit our virtual Growth Direct booth and view our 2020 posters that were presented at PDA Micro 2020.