blog
December 16, 2020 | Source: Rapid Micro Biosystems, Inc.
Rapid Automation: The Gold Standard for Implementing Lean in the QC Micro Lab
POSTED BY Rapid Micro Biosystems | 10 minute read
December 16, 2020 | Source: Rapid Micro Biosystems, Inc.
POSTED BY Rapid Micro Biosystems | 10 minute read
Augmenting Lean principles with technology can take your QC micro lab to the next level of efficiency, safety, and profitability.
QC micro labs are under constant pressure to reduce costs while improving quality. To accomplish this, many QC leaders have been embracing the same Lean principles used in manufacturing. These principles originated in the Japanese automotive sector were used to find and eliminate waste across the process. When implemented with fidelity, Lean can also work well to eliminate waste within QC micro testing processes and the manufacturing process itself. Just like in manufacturing there are also similar processes in QC labs which can lead to human error, variable workloads, facility layout, shared equipment, and shifts between routine testing and specialized tests.
Attempts to eliminate these areas of waste through retraining, rescheduling, and redesigning the lab can be complex and frustrating, which is why technology that uses the Rapid Automation Method, like our best-in-class system Growth Direct®, is becoming the gold standard for standardizing and streamlining processes in the QC micro lab.
What is the Rapid Automation Method?
This method, which automates the QC micro testing process, utilizes technology to achieve greater efficiency with minimal human handling. Rapid automation allows QC micro labs to detect contamination earlier, improve flow of information for faster decision-making, reduce labor costs, and speed product and equipment release and availability. This system can also enable a paperless LIMS and ensure greater data integrity.
How the Rapid Automation Can Lean Your Lab
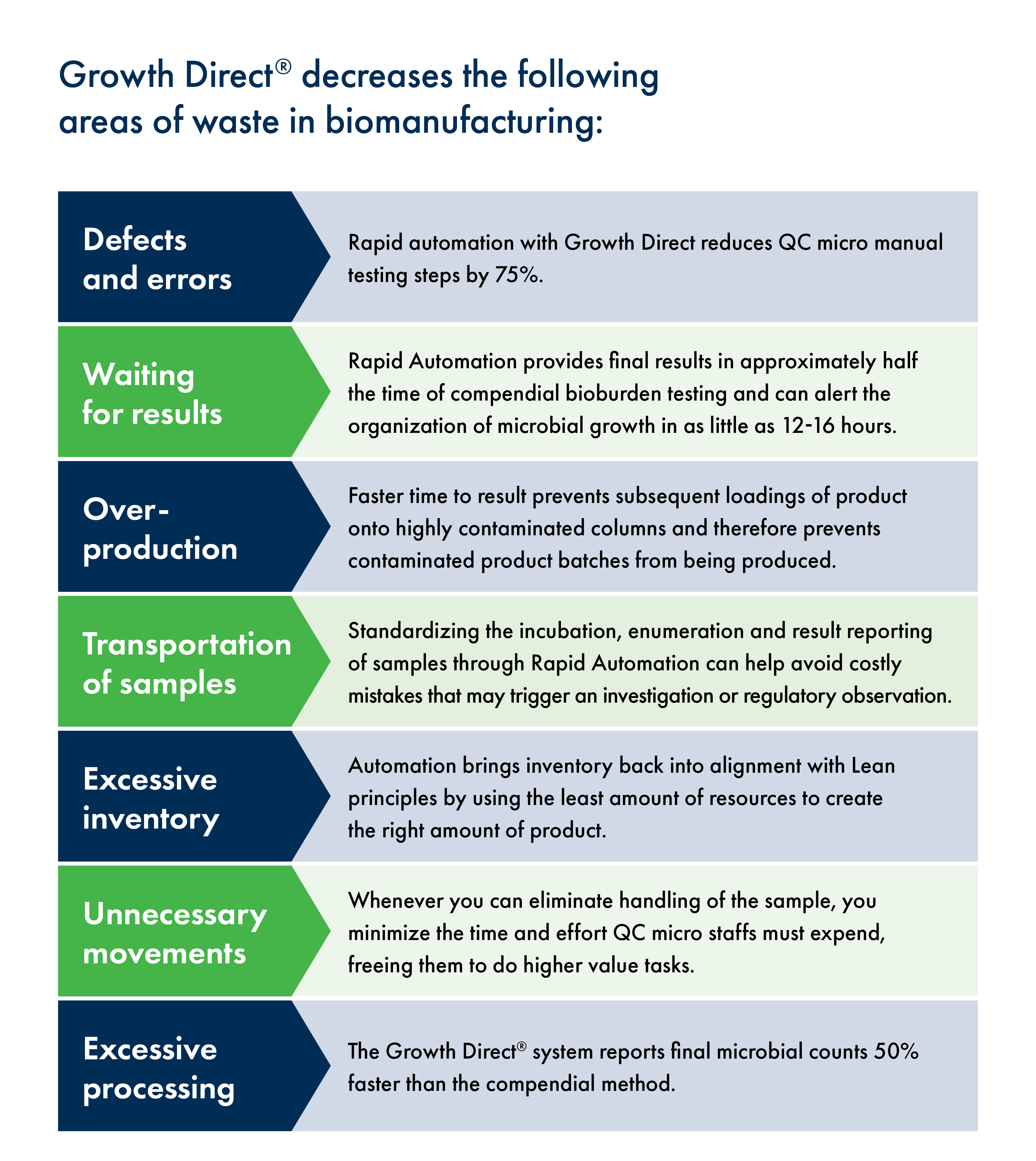
Layering the Rapid Automation Method over Lean principles to optimize processes in the lab can reduce the burden of the top seven major areas of QC lab waste, which generally include:
It is sometimes possible to minimize these areas of waste by retraining technicians and redesigning the workspace, but many QC micro labs leaders do not have the time or resources to continually assess and update processes or retrain staff, and some of the waste created in QC micro testing is due to the length of traditional tests themselves.
Automating the QC micro testing process results in fewer manual tasks, fewer errors, fewer regulatory issues, and fewer validation requirements. It also frees up skilled staff so they can focus their expertise on higher value functions.
Defects and Errors
80% of product quality issues occur due to human errors, which are difficult to track and almost impossible to fully eliminate. Those errors often lead to FDA citations and/or investigations that can cost thousands and even millions of dollars, making this one of the greatest sources of potential waste in the QC micro lab.
To mitigate these risks, some organizations deploy dual verification for all sample counts and transcriptions. This is extremely time consuming and costly and relies on a 16-step manual process performed thousands of times to reach 100% compliance. QC Micro teams, like any team, face turnover and at various points labs may be relying on team members with limited experience in an audit setting. Rapid automation eliminates 75% of manual testing steps, provides verification by exception, and removes any of these risks in an FDA audit.
Waiting for Results
Growth Direct Rapid Automation provides final results in approximately half the time of compendial bioburden testing and can alert the organization of microbial growth in as little as 12-16 hours. Early notification will significantly reduce the impact, remediation needs, and financial costs associated with microbial contamination events.
Over-Production
Faster time to result can prevent subsequent loadings of product onto highly contaminated columns and therefore prevents contaminated product batches from being produced. Ultimately, this eliminates the costs of over-production due to poor quality product.
Contamination events do happen, and industry itself reports that the average rate of batch failure in biopharmaceutical manufacturing due to contamination is 1.99% . Earlier detection of contamination allows for earlier remediation and faster return to normal production levels, providing greater flexibility and reducing risks related to supply chain.
Transportation of Samples
A Rapid Automation platform that uses a barcode labeling system for samples can improve workflow and reduce sample transport by utilizing standardized labeling for the cassettes that are loaded into a machine. Labels provide instructions for the automated system about how to incubate and handle the samples and enables the lab to track samples as they move through the process. Carrying carousels secure transport of the samples to and from the machine and reduce the risk of dropped samples.
Standardizing the incubation, enumeration and result reporting of samples in this way can help avoid costly mistakes that may trigger an investigation or worse a regulatory observation. The image below is an example of what can happen when these processes, which are vulnerable to human error, break down. Regulators are highly attuned to the risks of manual processes, and they are actively targeting breaches caused by them, because images like this one are unacceptable.

Excessive Inventory
Inventory costs at a biopharmaceutical facility are vast, and burdensome inventory represents a significant opportunity for improvement. These costs include manufacturing raw materials, manufacturing materials, quarantined in-process materials, and quarantined “finished” product.
A Rapid Automated system that is connected to LIMS can control costs in four ways:
Unnecessary Movements by Technicians
By removing the majority of human interaction with a sample, Rapid Automation enables full sample security and ensures error-free incubation, enumeration, and result reporting. In addition, whenever you can eliminate handling of the sample, you minimize the time and effort QC microbiology staffs must expend, freeing them from mundane tasks so they can do the higher value tasks that allow them to make continuous improvements in efficiency and operations.
A key area of waste in the original Lean principles is Muri, which is defined as burdensome pressure on employees and equipment. Reducing unnecessary steps such as traveling across the lab for a petri plate or across the campus of a major manufacturing facility to retake a sample and transport them back reduces pressure on lab technicians and can improve employee satisfaction, which also drives cost savings in the long run.
Excessive Processing
Rapid Automation streamlines the incubation, enumeration, sample sorting, and reporting for EM, Water, and Bioburden samples. The system eliminates the need for additional labor for secondary verification of samples put on this system. In addition to enhancing the robustness of data integrity in the QC Micro lab, the Growth Direct system reports final microbial counts 50% faster than the compendial method and eliminates 75% of the steps of manual microbial testing.
Ultimately, digitizing and automating the QC micro lab processes are essential for Lean practices for both the manufacturing execution system (MES) and the quality management system (QMS) to improve operations and mitigate unnecessary waste and keep costs low. Augmenting those practices with technology such as the Growth Direct can help standardize behavior and expectations across the lab while eliminating manual steps that can lead to variation in processes, burdensome procedures, and increased production timelines.
To learn more about lean initiatives through Rapid Automation, check out this on-demand webinar hosted by Kevin Walsh, Technical Services Manager at Rapid Micro Biosystems.