blog
July 30, 2018 | Source: Rapid Micro Biosystems, Inc.
Growth Direct™ Passes Rigorous Analytical Validation
POSTED BY Rapid Micro Biosystems | 2 minute read
July 30, 2018 | Source: Rapid Micro Biosystems, Inc.
POSTED BY Rapid Micro Biosystems | 2 minute read
At this year’s PDA Europe Annual Meeting, held in Berlin, June 26-27, 2018, Rapid Micro Biosystems delivered the poster presentation “Primary Validation for an Automated Colony Counter per European Pharmacopeia 9.2, 5.1.6.”
The poster details how the Growth Direct™ System complies with the comprehensive manufacturer primary validation assay performance requirements defined in the recently revised European Pharmacopeia 9.2 Chapter 5.1.6.
Growth Direct Satisfies Comprehensive EP 5.1.6 Validation Requirements
To ensure that Growth Direct complies with the directives required by the Europe Pharmcopeia for primary validation, Growth Direct underwent multiple rigorous tests. The data demonstrate that, when performing the analytical validation defined by the European Pharmacopeia, the Growth Direct complies with the assay performance requirements defined in the EP Ch 5.1.6. Equivalence was demonstrated with the compendial method using media from two alternative suppliers: Millipore and BD for both bioburden and environmental testing formats.
Impressive Colony Count Accuracy Excellent Organism Recovery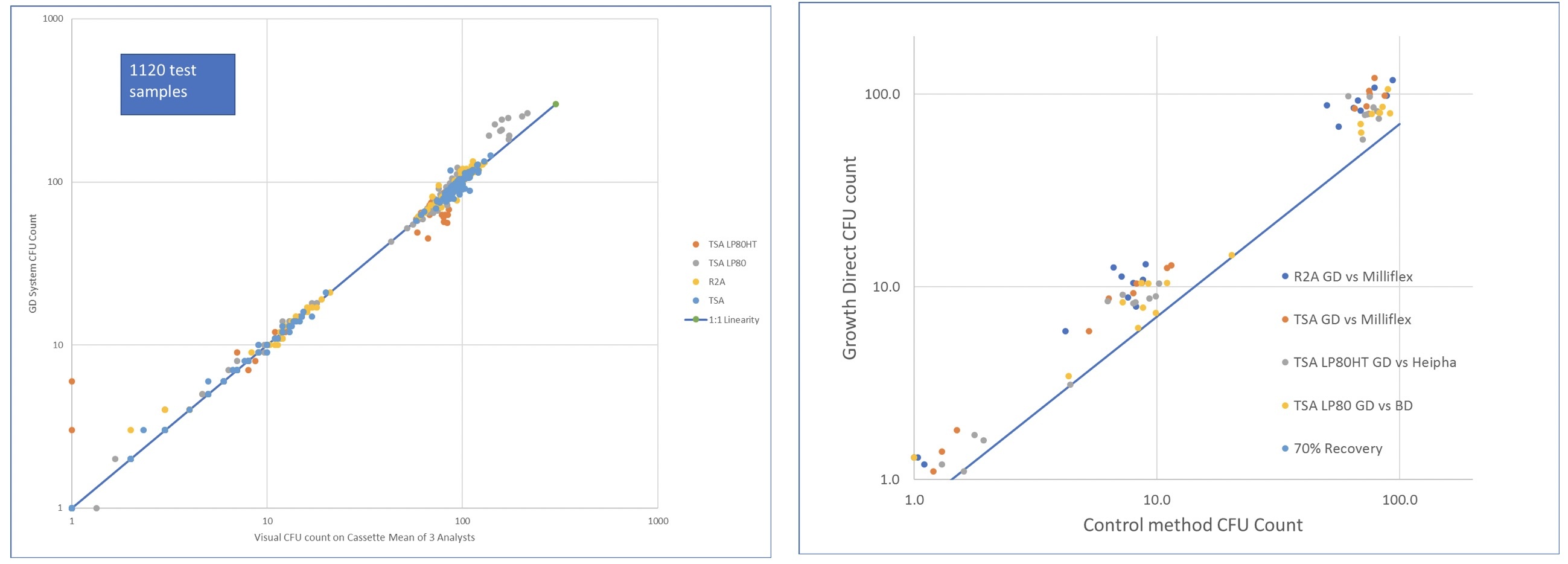
Conclusion: The PDA Europe poster shows a successful analytical validation of the Growth Direct. This validation is further supported by numerous systems being successfully validated at Top 20 Pharma companies and audited by regulatory bodies while in routine use at those sites.
To read all results reported in “Primary Validation for an Automated Colony Counter per EP 9.2, 5.1.6,” click here.
To learn how Growth Direct improves the accuracy, speed, efficiency, and productivity of your microbiology QC lab.