blog
June 16, 2020 | Source: Rapid Micro Biosystems, Inc.
Understanding the Most Efficient and Effective QC Testing Methods for Your Lab
POSTED BY Rapid Micro Biosystems | 6 minute read
June 16, 2020 | Source: Rapid Micro Biosystems, Inc.
POSTED BY Rapid Micro Biosystems | 6 minute read
Are Rapid Microbial Methods (RMMs), Automated Microbiological Methods (AuM) and Alternative Microbiological Methods (AMMs) the same? Can the terms be used interchangeably?
The short answer is no.
But that doesn’t stop our industry as well as many regulatory and compendial documents from linking these methods together.
It’s imperative that we as QC professionals on the frontlines understand how these terms are different and how each may benefit our process and lab environment. By gaining a clear understanding of the differences between rapid, alternative, and automated microbiology methods, we can align what needs to be changed in our labs, as well as when, where, and why. It will also enable us to use the proper terminology more consistently in regulatory submissions.
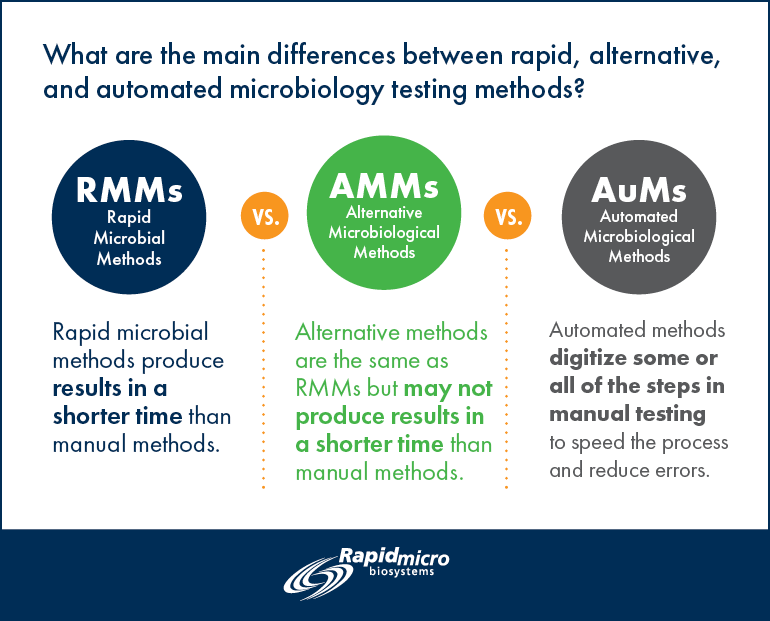
Rapid Microbiological Method
Definition: A novel or modern microbiological testing method that is different from classical methods and produces results in a shorter time.
A Rapid Microbiological Method may utilize instrumentation and software to manage the testing and resulting data. The results obtained may be qualitative, quantitative or identification of a microorganism.
The ultimate rapid method tests are completed in real time but may still be susceptible to human error.* To be used in replacement of compendial methods, the RMM must be at least as good as (i.e., non-inferior to the classical method) the classical method and it must be validated. Examples of RMMs include the real-time viability counters used for air monitoring.
Note: If you are changing the sample size for the test, be sure that you have validation testing showing that the different sample size testing is not inferior to the classical method’s sample size.
Alternative Microbiological Methods
Definition: A modern microbiological testing method that may utilize instrumentation and software to manage the testing and resulting data. It is different from a rapid method in that it may not achieve the results in a shorter time. This method is susceptible to human error.* The results obtained using AMMs may be qualitative, quantitative or identification of a microorganism. To be used in replacement of compendial methods the AMM must be at least as good as (i.e., non-inferior to the classical method) the classical method and it must be validated as an alternative method. For example, SCANRDI® is an AMM.
Note: If you are changing the sample size for the test, be sure that you have validation testing showing that the different sample size testing is not inferior to the classical method’s sample size.
Definition: Automated Methods (AuM)
Definition: Automated methods reduce the many steps of manual testing through the extensive use of instrumentation and software technology. To be used in replacement of compendial methods the AuM must be at least as good as (i.e., non-inferior to the classical method) the classical method and it must be qualified. Typically, this type of system does not need as much validation data as RMMs or AMMs. Rapid Micro Biosystems’ Growth Direct™ is an example of an AuM.
*Test methods vary by the degree of human manipulation required so human error can remain.
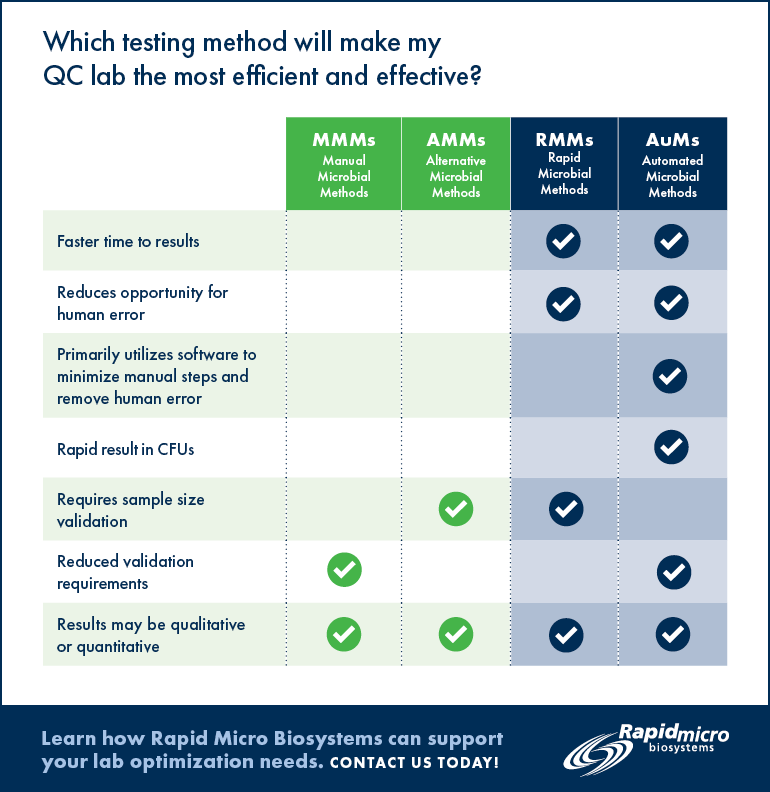
Which testing method will make my QC lab the most accurate and efficient?
The following chart may help you decide which method is right for your lab.
What Now?
Understanding the differences between microbiological testing methods can help you identify the type of testing you require. It can also aid in setting different requirements for validation/qualification for different types of systems. For example, 21 CFR 314.70 states, “Supplements and other changes to an approved application,” the addition or deletion of an alternate analytical method does not require prior approval and may be filed in the Annual Product Report.
If you and your team need further support in evaluating these methodologies and which one may make your lab more efficient and effective, please contact us for a consultation.
Related articles:
Podcast: Maintaining Operational Efficiencies in Micro Labs During a Pandemic
QC Labs That Employ Rapid Microbiological Methods Are Better Positioned to Endure the COVID-19 Pandemic