blog
April 10, 2024 | Source: Rapid Micro Biosystems, Inc.
Aligning Automated Bioburden Testing and Regulatory Policy
POSTED BY Rapid Micro Biosystems | 8 minute read
April 10, 2024 | Source: Rapid Micro Biosystems, Inc.
POSTED BY Rapid Micro Biosystems | 8 minute read
The United States Pharmacopeia (USP) recently proposed two new regulatory chapters – USP <1119> and <1119.1>. These signify a significant shift in the regulatory landscape, aiming to enhance bioburden testing practices and ensure consistency and reliability across the pharmaceutical industry.
Read Part 1 to learn more about the changes in USP regulations
Both current and proposed regulatory chapters for bioburden testing describe three main test options for colony enumeration: membrane filtration, pour-plate, or the surface-spread method. Membrane filtration is an established and reliable method for fast, flexible testing, especially for samples with low microbial loads or when specific organisms need to be isolated and identified.
Each of these techniques relies on visual plate counting as the primary method for quantitative microbiological analysis. While this approach is highly sensitive, reliable, and simple, it relies on counting visible colony growth, which can be challenging due to slow-growing organisms and the potential for human error with manual counting. These constraints can have significant implications during product development, including batch loss and failure to comply to regulations.
It’s no surprise then that there is a growing trend towards rapid microbial methods (RMMs) that offer faster and more accurate techniques for colony enumeration and reporting. This means a faster time to result, giving you the opportunity for better control over the manufacturing process – including making timely interventions and shortening the time-to-market for pharmaceutical products with limited shelf lives.
Global regulatory bodies, like the FDA and EMA, have assessed various rapid bioburden testing systems. Meeting all acceptance criteria, one of these is the Growth Direct® System which is designed to automate the incubation of bioburden media plates and enumerate any colonies present on the media [1]. This system follows the same procedures as membrane filtration; recommended as one of the most accurate bioburden testing methods in USP chapters <61> and <1119.1>, while delivering minimal hands-on time.
A key differentiator and advantage of the Growth Direct over traditional membrane filtration is its ability to detect colonies several generations before they become visible to the naked eye (Figure 1).
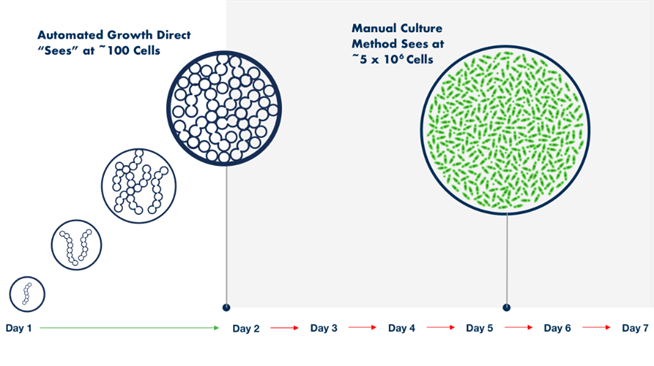 Figure 1. The Growth Direct® System automates the incubation and reading of test samples, detecting microbial growth at a much earlier stage of colony development.
Figure 1. The Growth Direct® System automates the incubation and reading of test samples, detecting microbial growth at a much earlier stage of colony development.
By combining digital imaging technology with sophisticated software algorithms, you can use this system to detect the cellular autofluorescence of growing colonies much earlier than by eye, significantly reducing turnaround times and eliminating the need for manual counting (Figure 2).
The Growth Direct® System halves the time spent on bioburden testing. It automatically counts colonies every four hours and migrates results to LIMS electronically, automating the workflow and streamlining data management.
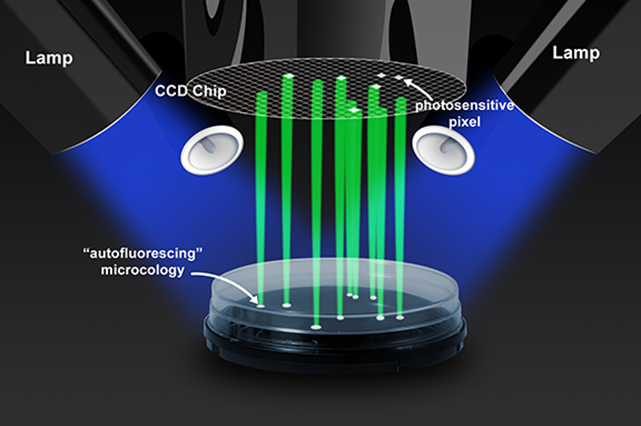 Figure 2. By combining digital imaging technology with sophisticated software algorithms, the Growth Direct® System can detect and count the autofluorescence of growing microbes with accuracy and consistency.
Figure 2. By combining digital imaging technology with sophisticated software algorithms, the Growth Direct® System can detect and count the autofluorescence of growing microbes with accuracy and consistency.
How does the Growth Direct® System align with regulations?
The Growth Direct® technology is based on the compendial method outlined in USP <61> and <1119.1>, employing industry standard media, reporting results in CFUs, and replicating the current sample preparation. This ensures seamless compliance with current and future regulations, making the validation process for this method exceptionally straightforward.
As a result, the USP <1223> and the European Pharmacopoeia (EP) chapter 5.1.6 classify the Growth Direct® System as an automated compendial method, requiring only a verification of the counting method followed by a method suitability study [2]. Adhering to this, the Growth Direct® System has undergone a simplified verification approach for automated incubation and enumeration of microbial colonies obtained from in-process bioburden testing [3]. This validation showed that the Growth Direct® System:
Read the detailed validation of the Growth Direct® System for bioburden analysis
As the pharmaceutical industry continues to evolve and develop new and complex biological products, it's clear that regulations will need to adapt and ensure that QC measures keep pace with innovation. The ever-changing landscape demands a proactive approach from manufacturers to maintain compliance and uphold product safety and efficacy standards.
Automated systems like the Growth Direct® offer a promising solution, providing faster, more accurate, and reliable methods for bioburden assessment; all while minimizing hands-on time. By embracing these advancements, you can streamline your testing procedures, reduce the risk of human error, and accelerate the release of pharmaceutical products to market.
Request a demonstration today to see how the Growth Direct® system can benefit your facility.
References