blog
September 11, 2023 | Source: Rapid Micro Biosystems, Inc.
Ask Rapid: Extending Your Countable Range
POSTED BY Rapid Micro Biosystems | 10 minute read
September 11, 2023 | Source: Rapid Micro Biosystems, Inc.
POSTED BY Rapid Micro Biosystems | 10 minute read
If your QC Microbiology lab is hampered by overpopulated test plates, “Too Numerous to Count” (TNTC) results, or time-consuming dilution prep, this edition of “Ask Rapid” is for you. Because we’ll explain why automated colony counting with the Growth Direct® System offers a flexible yet more precise alternative.
“We often hear ‘how high can you count’ questions about our technology,” notes RMB Field Application Scientist Johannes Oberdörfer. “The questions usually come from industries with intrinsically high counts – foods and beverages, non-sterile manufacturing, medical devices, or sterile manufacturing operations where in-process and water samples exhibit high counts at certain product stages.”
One area of need is preservative efficacy testing (PET), used to confirm that antimicrobial additives can retain their effectiveness over time. Labor- and resource-intensive, PET requires numerous manual manipulations to obtain an accurate estimate of the bioburden of test samples over a 28-day period. In this as in many other QC Micro activities, the traditional compendial method can introduce needless testing delays due to the human eye’s limited ability to quantify colony forming units (CFU).
While individual company practices vary, a general rule of thumb for more than a century has been Petri plates yielding no more than 300 CFU, with an upper range of 250 CFU when using the smaller contact plates for environmental monitoring (EM). Once there are too many colonies, it becomes difficult and time-consuming for microbiologists to visually distinguish individual colonies.
“When the traditional plating method runs into trouble with excessive CFU counts, the obvious response is to dilute the samples so you can get within a countable range,” explains Oberdörfer. “If you’re getting bioburden counts greater than 1000 CFU, for example, you might need to dilute 1:10 or 1:20 to get within a countable range. But this comes with significant drawbacks.”
The drawbacks include:
In contrast, the Growth Direct® System replaces human observation with optical imaging and algorithmic vision analysis. Our technology can detect microbial colonies with as few as 100 cells, representing a level of sensitivity approximately 50,000 times greater than the human eye. This supports a more robust counting range, yet still meets USP <1223> conditions for an automated system of traditional microbial plate counting that “allow[s] colonies on solid media to be read more quickly, with substantially less incubation time than is possible using only the unaided eye.”
LEARN MORE
Ask Rapid: Understanding Algorithms
Validation of the Imaging Technology for a Novel Microbiological Colony Counter
To show the enumeration range of the Growth Direct® System, let’s look at data from RMB’s 2017 validation studies on the four key media types for pharmaceutical quality control testing (TSA, R2A, TSA LP80, and TSA LP80HT).* Seven organisms were evaluated, including the five USP standards and two others of interest, E. coli and S. epidermidis. A mixed culture was also run, Candida albicans and Bacillus subtilis.
The data is shown graphically below for the combined data for the range of organisms tested. The x-axis uses the target CFU dose number for each dilution. The y-axis uses the actual Growth Direct® System CFU result calculated from all the replicates on all four media, giving a total of 40 data points for each test dose.
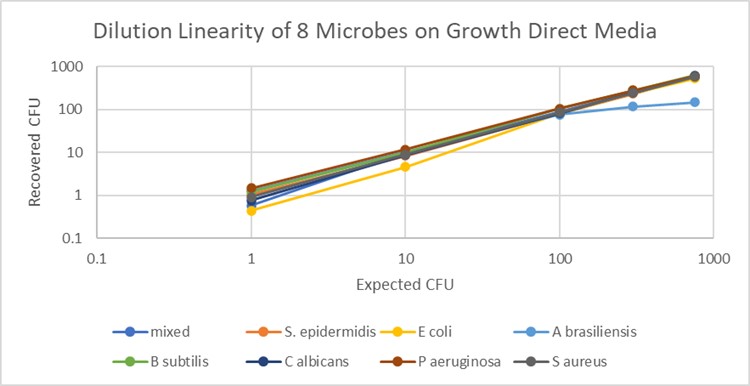
Notes on the study: Where available, high-dose bioballs were used to create the range of organism concentrations at five levels from 750 to 1 CFU. Ten replicates were made for each dose, and either spread-plated to the two EM media or prepared after membrane filtration for the bioburden media. Incubation was for 3 days at 30-35°C in the Growth Direct® System. At the end of incubation, the mean of the 10 reps was calculated for each organism and each media. The mean result was then calculated across the four media for each organism.
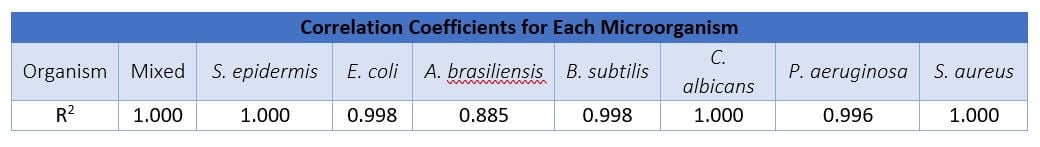
The data shows that the Growth Direct® System can linearly enumerate organisms over the range of 1 to 750 CFU, in both EM and bioburden assay formats. (The one exception, A. brasiliensis, has a well-known lower counting range due to the rapid spreading mode of growth. On the Growth Direct® System, the linear counting range is wider than the manual count up to 100 CFU.) Accurate enumeration of microorganisms over a 4 log counting range allows operators to reduce the number of dilutions required to obtain a result for those applications where high CFU counts may be expected – for example, the preservative efficacy test as defined in USP Ch <51>.
In this test a high dose of the test organism, >105 CFU, is added to the test material containing the preservative and the surviving organisms tracked over a 28-day period. USP <51> specifies the expected reduction in cell numbers with time. As the cell number could be anywhere between 1 and >105, a number of sample dilutions are required to ensure that the count falls in the accurate counting range for the compendial test. Use of the Growth Direct® System could reduce the number of dilutions by 50% – thereby reducing manipulation time and potential dilution errors.
A secondary benefit to the use of the Growth Direct® System for this application? Earlier time-to-result (TTR).
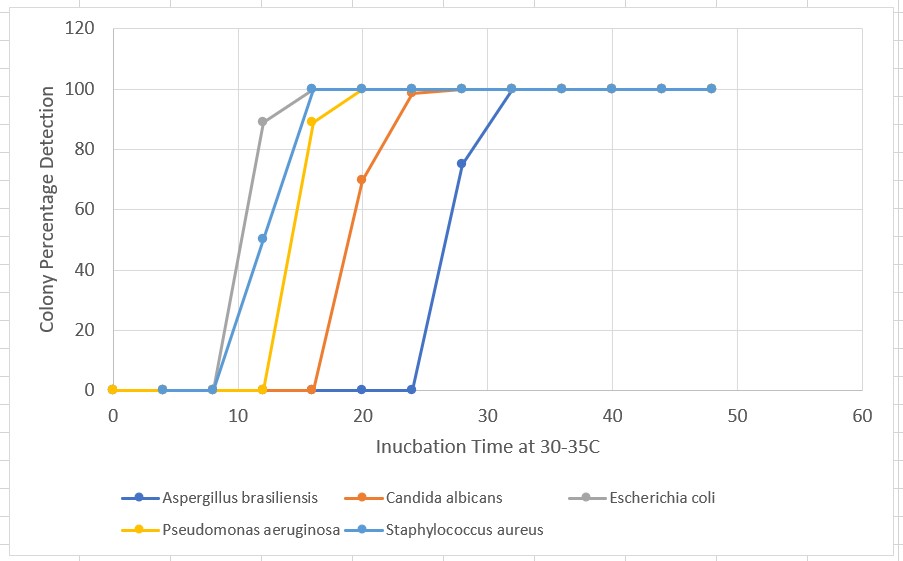
For the five organisms defined in USP <51> (A. brasiliensis, E. coli, S. aureus, C. albicans, P. aeruginosa), quantitative enumeration can be obtained in <24 hours (TBC still valid). The graph below shows the cumulative colony detection curves for the organisms of interest incubated on TSA media at 30-35°C. Maximum number of detected colonies occurs earlier than 24 hours.
If your QC Micro lab still relies on manual colony counting and could benefit from greater quantification capability, the Growth Direct® System combines a robust alternative with relatively straightforward implementation. In addition, test results can often be obtained 50% earlier or better, depending on your current method. That means swifter release of product.
As an automated compendial approach, the Growth Direct® System only requires the assurance of accuracy, precision, and equivalence as compared to the reference standard for validation; it has been successfully reviewed with the FDA and EMA and is in routine GMP use by multiple companies in the United States and Europe. Equally important, Rapid Micro Biosystems’ highly experienced validation team can provide a complete suite of validation documents, services, and project management to ensure timely qualification – often in less than six months.
Have more questions? Contact Rapid Micro Biosystems today.
*Internal data on file
Preservative Efficacy Testing Through the Growth Direct® System
Primary Validation of the Growth Direct® Bioburden System and Media
Validation of the Growth Direct® System to Perform Pharmaceutical In-process Bioburden Analysis