blog
November 14, 2023 | Source: Rapid Micro Biosystems, Inc.
Common Fungal Contaminants and Their Effects
POSTED BY Rapid Micro Biosystems | 5 minute read
November 14, 2023 | Source: Rapid Micro Biosystems, Inc.
POSTED BY Rapid Micro Biosystems | 5 minute read
Bacterial contaminants are the most common concern of pharmaceutical manufacturers, but fungal contaminants also pose very significant threats. With effects that range from product spoilage to high mortality rates among immune-compromised patients, fungal contamination has frequently led to infection outbreaks and product recalls in recent years.
The group Fungi contains both yeast and filamentous mold. While mold is rarely seen as a contaminant in Class A/B cleanrooms (in < 1% of Class C/D samples), it can be a significant issue due to the generation of spores that can rapidly contaminate a larger area. A review of product recalls from 1998 to 2006 reported that 23% were due to yeast and mold for non-sterile products, and 7% were found in sterile products.
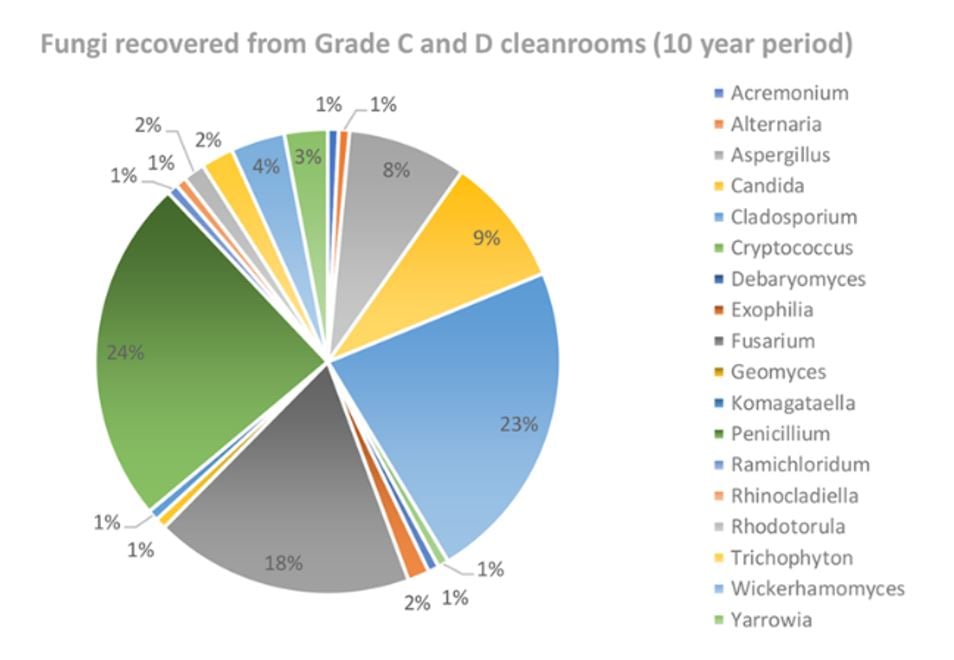
The most frequent fungal contaminants, identified by Tim Sandle and shown in Figure 1 below, included Fusarium sp., Cladosporium sp., and Penicillium sp., with Aspergillus sp. close behind.
FIGURE 1: Reported frequency of mold species found in manufacturing suites over a 10-year period, incubated on TSA-based media at 20°C-25°C, followed by 30°C-35°C.
A number of companies have reviewed the most frequent 483 citations given to pharmaceutical manufacturers by the U.S. Food and Drug Administration (FDA). One issue often placed among the three most common violations is poor investigation of root cause and correction of the discrepancy. This issue was most graphically brought to light with the major mold contamination of sterile parenteral products at the New England Compounding Company (NECC), where mold contamination was detected but investigation and correction were not performed satisfactorily.
Contamination Fears Pose Big Challenges in Aseptic QC
Same-Day Mold Detection Becomes Reality
Implementation of a rapid microbial method (RMM) can significantly reduce the time to detection of an environmental contaminant and facilitate a faster response. Automated colony counters have gained acceptance by the major global regulatory bodies and can reduce the time to detect a microbial contaminant (bacteria and mold) to <24 hours.
In fact, recent enhancements to the Growth Direct® System now allow a growing colony to be differentiated into mold or bacteria to give more information early to facilitate remedial action.
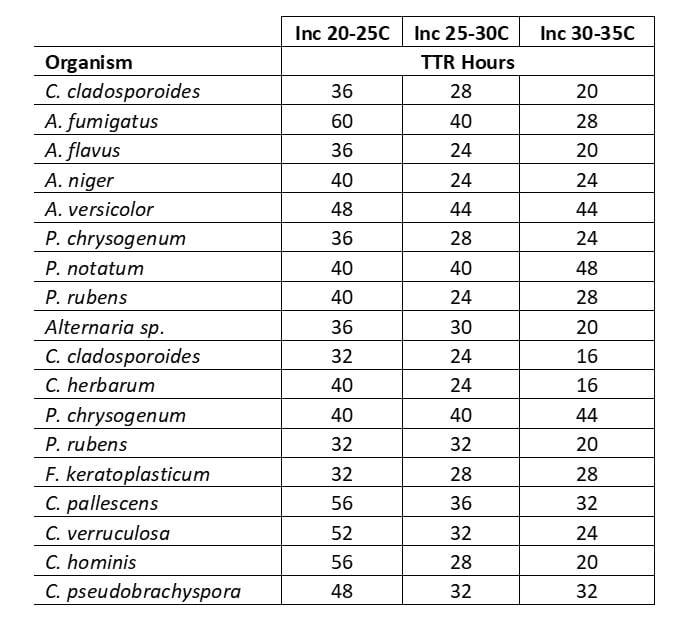
Table 1 shows the maximum incubation time to confirm the quantitative presence of a mold, including time to result (TTR) when incubated at one of the range of temperatures used for environmental monitoring (EM) testing. Sensitivity to incubation temperature varies by organism type but in all cases the result is confirmed in ≤60 hours. Detection timescales are similar for bacterial contaminants found in manufacturing environments.
TABLE 1: Maximum Incubation Times for Mold Confirmation, Growth Direct® System
Bacterial and fungal contamination in aseptic production environments have a critical impact on patient safety and manufacturing costs, while posing serious risks to supplier reputation. In the case of mold detection, current compendial workflows typically require a microbiology staff to wait until the end of sample incubation before manually verifying the presence of mold colonies. Even when performed expertly, these labor-intensive processes make it challenging to head off the spread of mold contamination before it compromises a facility.
RMBNucleus™ Mold Alarm now offers an expedited alternative, enabling earlier confirmation of contamination and cleaning efficacy. This can result in a lower cost to return aseptic manufacturing facilities to a decontaminated state, while also reducing the risk of human error. To learn more, contact Rapid Micro Biosystems today.
Blog by David Jones, Director of Technical Marketing and Government Affairs at Rapid Micro Biosystems.