blog
April 30, 2018 | Source: Rapid Micro Biosystems, Inc.
Data Integrity and the Growth Direct™
POSTED BY Rapid Micro Biosystems | 5 minute read
April 30, 2018 | Source: Rapid Micro Biosystems, Inc.
POSTED BY Rapid Micro Biosystems | 5 minute read
Data integrity has always been an integral component of current Good Manufacturing Practices. In the past two years, the FDA and the Medicines and Healthcare Product Regulatory Agency (MHRA) have issued updated data integrity guidance. These documents have provided additional details on the agencies’ expectations and requirements. Consequently in 2016 and 2017, inspections and cited data integrity deficiencies issued by both agencies increased significantly compared to the previous years. Fallouts of data integrity deficiencies may take years to resolve and can range from increased investigation and response costs, to decreased trust from the FDA, to product recalls, and/or delayed market entry costs[i]. The financial impact of data integrity infractions can reach well into the hundreds of millions of dollars as the following table illustrates from James Davidson’s article “The Real Cost of Poor Data Integrity in Pharmaceutical Manufacturing.”
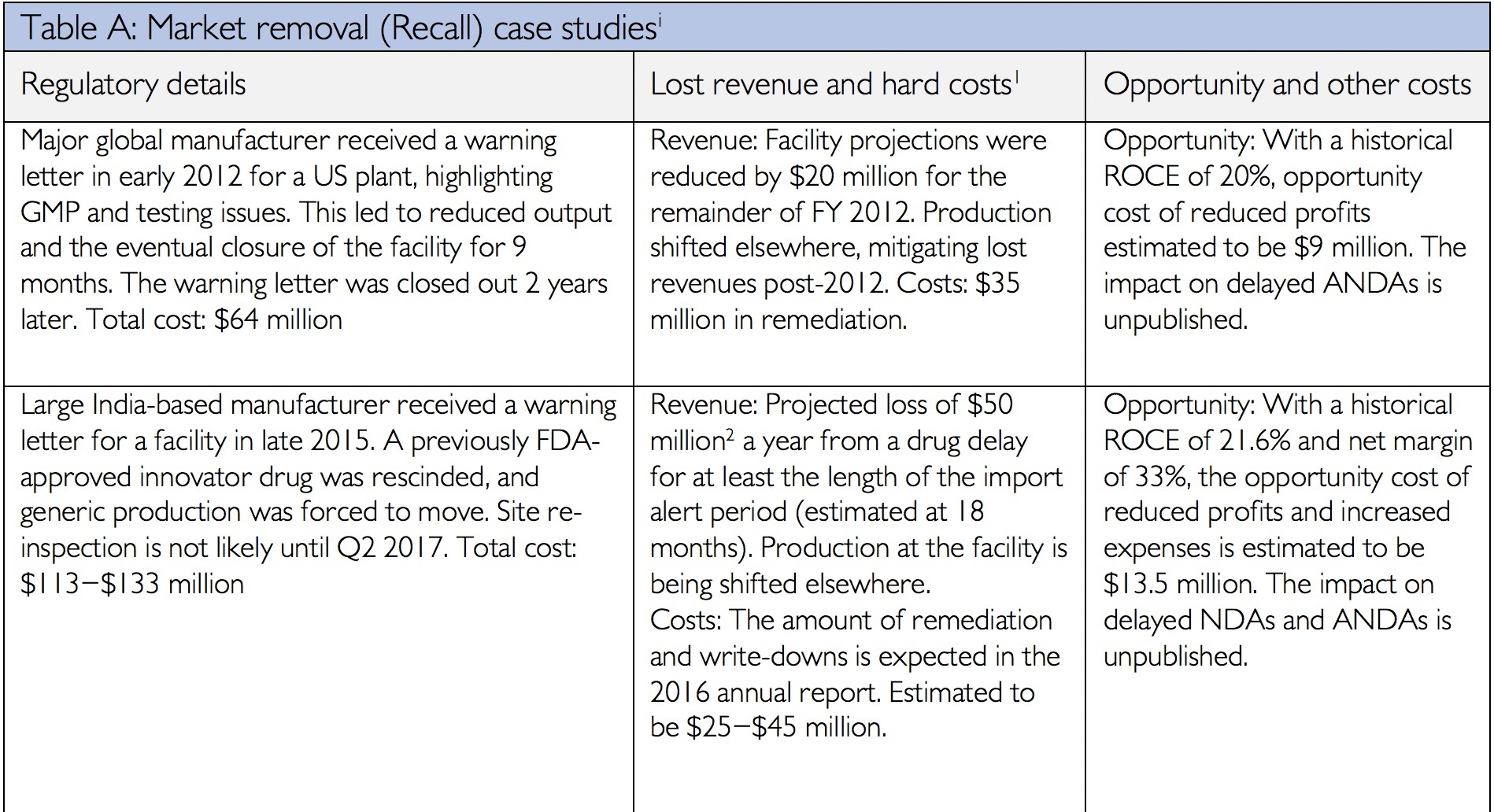
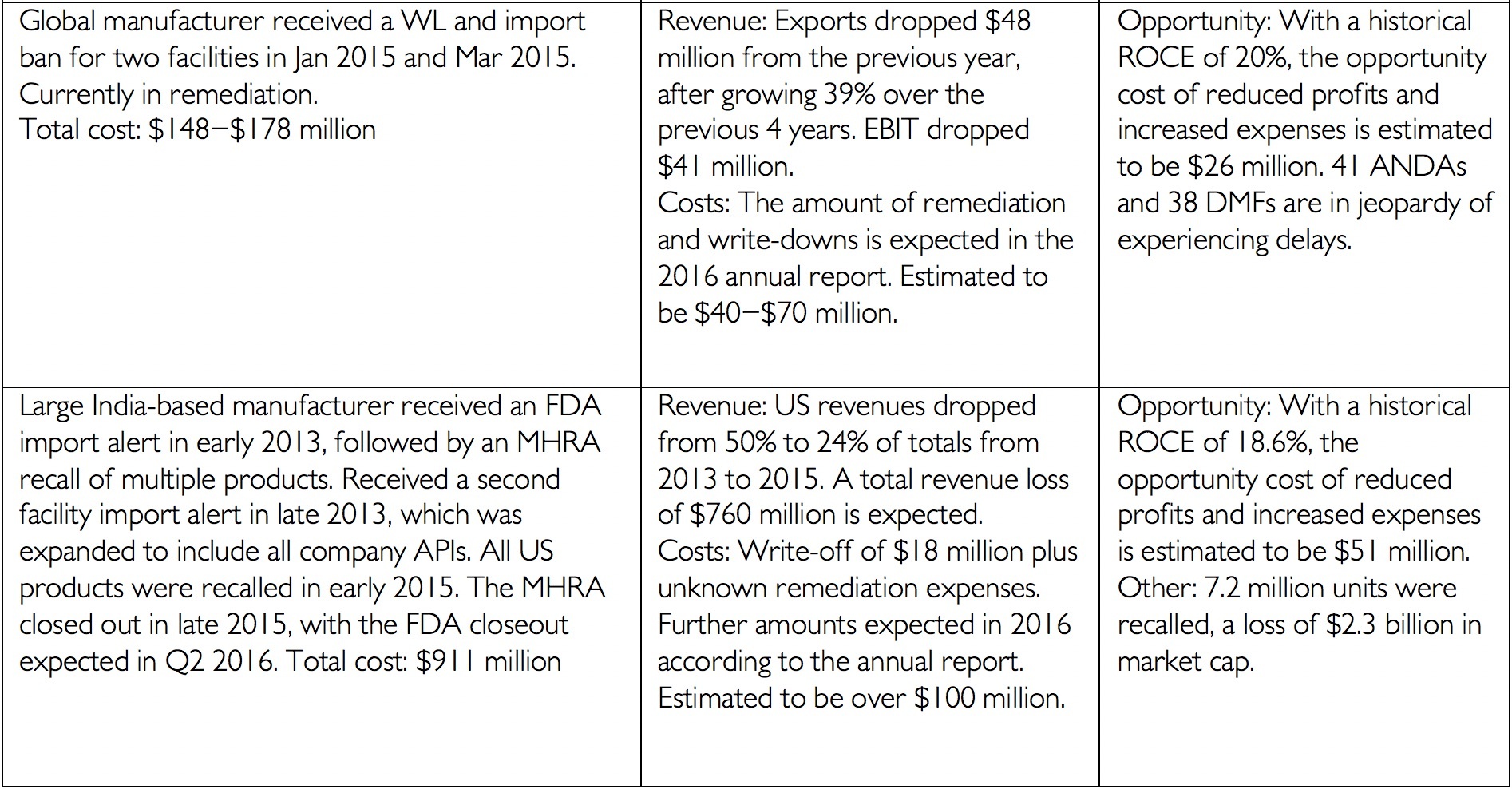
[i] Financial details from company annual reports for period in question
[i] In some cases, securities analyst research was reviewed where figures were unavailable. Securities firm names withheld to protect confidentiality.
Regulatory bodies like the FDA and MHRA have a long history with data integrity concerns, one that stretches as far back as the 1970’s. In the 1990’s and 2000’s, data integrity guidance documents focused on digital records, but now the focus on human generated data has re-emerged. Auditors are now asking for records of secondary verification of microbial counts done by the human eye. Data experts note that fraud does not cause most data integrity cases; in most cases, it is “sloppy practices” or “poor systems designs[i].” Some experienced microbiologists point to better training as the solution, not secondary verification. In 2018, that position has fallen out of favor, and multiple regulatory guidance documents[ii] and audit manuals[iii] now include specific examples of secondary verification of manually generated data including microbial counts.
This expectation of secondary verification has led to increased pressures on the QC Microbiology lab for more personnel to perform counts, write the protocols for what to do when counts differ and adjudicate discrepancies. Also, there is the added requirement for space – companies are asking where are we going to keep all these plates and will additional growth occur while we wait for a reviewer to see if the counts differ. Rapid Micro Biosystems’ Growth Direct system eliminates the need for these additional resources as it securely records the sample results in a password protected database, and all actions require individual authentication.
The Growth Direct optimizes data integrity in three key ways:
By automating the incubation and enumeration of QC Micro testing with the Growth Direct system, companies can proactively address the data integrity of their QC Micro lab and avoid both costly 483s and warning letters. For more information on data integrity and the Growth Direct click here, or to see how the Growth Direct can be combined with a QC Micro specific LIMS system click here.
[i] J. Davidson, “How to Avoid Data Integrity Disasters in Your Manufacturing Network.”