blog
July 30, 2020 | Source: Rapid Micro Biosystems, Inc.
Designing the High Performing QC Lab
POSTED BY Rapid Micro Biosystems | 8 minute read
July 30, 2020 | Source: Rapid Micro Biosystems, Inc.
POSTED BY Rapid Micro Biosystems | 8 minute read
How Taking an Honest Look at Your Lab Can Improve Speed, Capacity, and Agility to Help You Keep Pace with Progress

QC Directors and QC Managers are charged with maintaining and improving QC micro lab efficiencies, cost-effectiveness, and product quality. They must focus on operational issues such as ensuring their methods, process, and equipment are current; maintaining compliance with regulations; and monitoring the cost and quality of testing. This is a lot to manage, but the most effective leaders occasionally pause to take a broader view of the total lab environment and the ways in which their labs may be under-performing. Doing this well can lead to innovative solutions that result in a higher performing QC lab and a more empowered team.
To support your lab performance analysis, we’ve outlined three areas that take priority in creating an optimized lab environment: speed, scale, and scope.
![]()
Speed
The biopharmaceutical industry is under constant pressure to increase the speed of manufacturing without sacrificing quality. However, many labs continue to run manual tests in sequence that can take anywhere from 5 to 14 days. Manual testing also increases the chance of human error, making it difficult to achieve maximum quality and efficiency. The COVID-19 pandemic has recently underscored the importance of speed and efficiency and finally convinced many drug and vaccine manufacturers that the time for digitization of manual processes is now.
How to Improve Your Lab’s Speed
Employing digitization for some or all processes can have an immediate impact on the speed of manufacturing. Rapid automation methods, which automate the entire process, can cut the time needed to complete a test by half, and it enables steps that are traditionally run in sequence to be executed in parallel, saving time and eliminating the risks of human error. Quality control measures that monitor workflows and detect microbial contamination will continue to be mission-critical, particularly in enabling increased speed and capacity.
![]() Scale
Scale
The scale of manufacturing is also radically changing. Commercial manufacturing of pandemic-related therapeutics and vaccines will no longer be measured in millions of doses, but in billions. Once the pandemic is over, drug and vaccine manufacturers that have successfully expanded their scale in safe and effective ways will have a clear advantage in the marketplace.
How to Improve Your Lab’s Scale
Rapid automation methods that replace slow, error-prone, manual QC processes allow manufacturers to confidently accelerate bioprocessing, maximize capacity, and reduce operational risk and downtime, all while maintaining the highest standards of data integrity. Such technologies can take your QC processes to the next level of sophistication and enable your team to meet regulatory requirements, sustain competitive advantage, and, ultimately, serve patient needs and safety.
![]() Scope
Scope
Biopharmaceutical manufacturing includes newer treatments like cell and gene therapy in addition to the historical products like monoclonal antibodies, vaccines, and tissues and blood products. The processes for these complex therapeutics demand new standards for automation, sterility, speed, and quality. Traditional microbial QC test methods are slow to yield results, labor-intensive, prone to human error, vulnerable to tampering, and are incompatible with the need to accelerate manufacturing workflows. Delayed issue identification and resolution can slow deployment of therapeutics to patients, while issues with data integrity can severely impact the bottom line and corporate reputation.
How to Improve Your Lab’s Scope
High-capacity automation effectively replaces the time- and labor-intensive manual approach. Test results are available in dramatically less time, enabling faster product release, greater capacity, and enhanced data integrity and security.
Our CEO, Rob Spignesi, is passionate about improving manufacturing efficiency and predictability—especially in the face of increasing uncertainty due to COVID-19. He says, “At the broadest level, rapid automation for microbial detection is part of a dramatic shift in the biopharmaceutical industry to improve and augment their manufacturing processes to meet the stringent requirements of the growing number of complex drug modalities like biologics, cell and gene therapies, and vaccines. In the current environment, the COVID-19 pandemic has only served to accelerate this movement to improve manufacturing in the drug industry. The shift was already underway, and the pandemic has pushed the biopharmaceutical industry to accelerate their decision making to improve conventional manufacturing strategies.”
Demand for automated QC solutions is growing rapidly, particularly among manufacturers of novel complex therapies. The majority of the top 20 biopharmaceutical companies have adopted the leading automated QC solution, the Growth Direct™ platform, from Rapid Micro Biosystems. This includes deployment of the platform in the commercial manufacture of the most innovative treatments, such as CAR T-cell therapies.
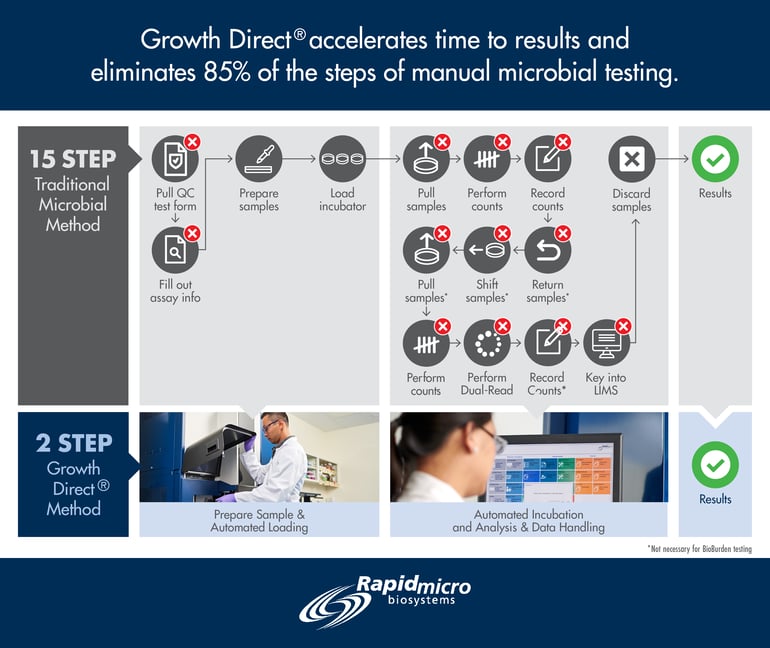
A cornerstone of this race to keep pace will be the integration of automated, high-throughput approaches to QC. This foundational technology will continue to pave the way for increases in speed and capacity, accompanied by the necessary risk mitigation, data integrity and patient safety, that will help reshape our industry for years to come, for the benefit of all.
Ready to uncover where your lab may be under-performing?
Download our Lab Performance Questionnaire today and get started. This simple 11-point survey will help you and your team begin to identify the most critical areas where your lab needs process and workflow improvement.
Other articles in the Lab Optimization Series:
Podcast: Maintaining Operational Efficiencies in Micro Labs During a Pandemic
QC Labs That Employ Rapid Microbiological Methods Are Better Positioned to Endure the COVID-19 Pandemic