blog
March 3, 2023 | Source: Rapid Micro Biosystems, Inc.
Empowering your GMP workflow with Quality Control Automation
POSTED BY Rapid Micro Biosystems | 2 minute read
March 3, 2023 | Source: Rapid Micro Biosystems, Inc.
POSTED BY Rapid Micro Biosystems | 2 minute read
The Pharmaceutical Microbiology Forum (PMF) recently added a whitepaper to their website that was originally written with analytical laboratories in mind, but several of the issues are relevant to microbiology laboratories. The purpose of the whitepaper is to outline common issues pertinent to GMP.
The paper is divided into six sections:
While each section dives into specific examples of issues arising within each and how to handle them, the underlying need addressed is the same: streamline processes to maintain control of the analytical laboratory environment to uphold GMP. Many of the issues arise with manual methods and processes prone to human error, so how does one empower the quality control (QC) microbiology laboratory to overcome these obstacles?
In a word, automation.
Automated rapid microbial methods (RMMs) eliminate unnecessary hands-on labor and manual data entry increasing the productivity of highly skilled technicians while reducing human-based errors, freeing them to use their expertise for high-impact activities while improving retention and reducing training costs.
Automated RMM vs Manual Method for QC Microbiology Testing (i.e. Environmental Monitoring, Bioburden, and Water Testing)
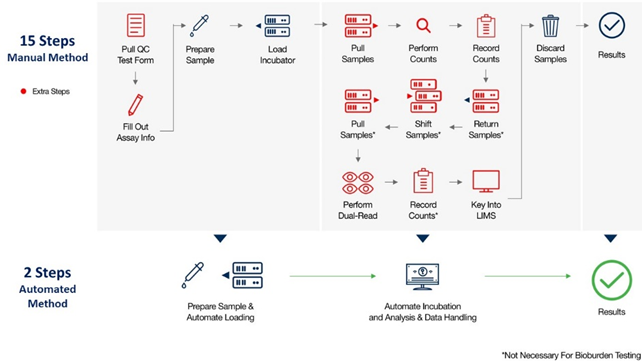
Automated RMMs deliver test results in dramatically less time, enabling faster product release, greater capacity, and enhanced data integrity and security, while eliminating inaccuracies resulting from human error.
To view the PMF whitepaper referenced above, click here.