blog
July 14, 2022 | Source: Rapid Micro Biosystems, Inc.
Glowing or Growing: Autofluorescence and the Growth Direct® System
POSTED BY Rapid Micro Biosystems | 7 minute read
July 14, 2022 | Source: Rapid Micro Biosystems, Inc.
POSTED BY Rapid Micro Biosystems | 7 minute read
Let's shed some light on a critical feature for biopharma companies choosing an automated colony counting system.
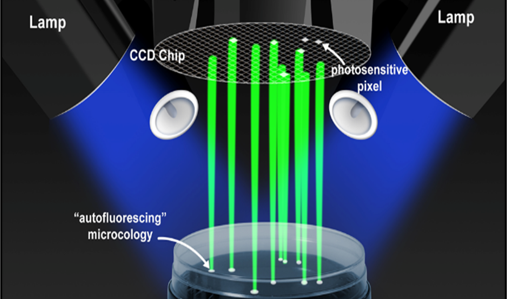
It started as a flash of insight. A small team of scientists, seeking a market for their proprietary imaging technology, came across some research on the natural presence of autofluorescent molecules in all living cells. And suddenly, they saw new synergies for pharmaceutical quality control.
The idea? Excited by a short pulse of blue light, viable microcolonies could actually announce their presence by radiating green fluorescent light, long before they were visible to the naked eye. As colonies grew bigger and brighter, automatic imaging and recording technology could be used to build a time-series and distinguish colonies from fluorescent debris. Such a technology could support microbial enumeration with a level of accuracy equivalent to traditional compendial methods, only in less time with less potential for human error.
Fast-forward twenty years, and our patented Growth Direct® System is now the most established automated colony counting technology in pharma QC micro.
As summarized in this new 14-minute webinar, research by the Biophorum Operations Group (BPOG) points to the following advantages for biopharma companies that successfully adopt automated colony counting systems:
But how do you choose the right system? While varied automated colony counters share some common traits, a defining difference of the Growth Direct® System is its reliance on intrinsic autofluorescence. If you are considering modernization of your QC Micro process with an automated colony counting system, that difference merits a closer look.
While cells must typically grow into the millions before the naked eye can detect them as a colony forming unit (CFU), the Growth Direct® System can see colonies with as few as around 100 cells. Inside its imaging station are multiple light emitting diodes (LEDs) that expose incubated system cassettes to blue light, causing the flavins in any living cell to radiate green fluorescent light. This autofluorescence is in turn detected by a large charge-coupled device (CCD) panel, similar to those used in astronomy photography, digital cameras, and other products requiring high-quality image data.
Allowing movement and manipulation of electrical charges, CCD sensors in the Growth Direct® System are divided into photoactive and transmission regions. When a photon (basically a tiny particle of light) hits the photoactive region, tiny arrayed capacitors accumulate electrical charges proportional to the intensities of light at their locations. These capacitors then pass their charges into an amplifier within the transmission region, which converts them to voltages. After those voltages are digitized and recorded to produce images, our proprietary imaging algorithms convert the light to grayscale pixel values and determine which groups of pixels might represent growing colonies.
Stimulated light emissions from autofluorescing microcolonies are detected by the Growth Direct® System's charge-coupled device (CCD) and converted into digital images for automated counting, after only a few hours of incubation.
Our imaging technology is growth-based and requires no reagents; samples are prepared identically to the traditional method, and all microbes are preserved during enumeration. When a cassette registers an out-of-specification (OOS) level of growth, microbiologists can quickly conduct further testing on its contents. Consequently, manufacturers can save days or even weeks on both colony enumeration and identification, and OOS investigations are far more likely to capture contaminants before they spread.
In essence, the Growth Direct® System simply automates the incubation and enumeration processes already in place at QC Micro labs worldwide. Final results are equivalent to the traditional method, and samples are preserved for microbial identification – but results are available in about half the time.
This leads to a second key difference between the Growth Direct® System and other automated technologies: broad regulatory acceptance.
Ours was the first technology to qualify as an automated system for the incubation and reading of the traditional plate count, based on conditions stated in USP <1223> and the 2013 PDA Technical Report No. 33. Consequently, it does not require full validation as an alternative microbiological method, merely verification of enumeration accuracy.
In contrast to rival systems only now being evaluated as part of the biopharma development cycle, the Growth Direct® System has been qualified and validated at multiple customer sites over the past seven years. Our technology has been implemented for cGMP processes for environmental monitoring and water testing through internal change control procedures, while being successfully implemented for in-process bioburden testing following successful license review by EMA and FDA.
Today there are many options for biopharma companies considering an automated colony counting system. If it all seems bewildering, just contact Rapid Micro Biosystems. We make the right choice easy to see.