blog
February 26, 2018 | Source: Rapid Micro Biosystems, Inc.
New Paper Describes the Simplified Validation Process of Growth Direct™ for In-Process Microbiological Testing
POSTED BY Rapid Micro Biosystems | 5 minute read
February 26, 2018 | Source: Rapid Micro Biosystems, Inc.
POSTED BY Rapid Micro Biosystems | 5 minute read
In-process microbiological testing can be both time-consuming and labor-intensive for the micro QC department. Reducing the Time to Result (TTR) and analyst involvement in sample analysis can help significantly. By automating the compendial process, the Growth Direct™ System from Rapid Micro Biosystems can help QC departments achieve both goals: reduced TTR and reduced analyst involvement.
A new paper entitled Validation of the Growth Direct System to Perform Pharmaceutical In-process Bioburden Analysis published in the Institute of Validation Technology, GXP Journal Articles, Vol. 22, Issue 1, Jan 2018, introduces the simplified validation strategy for the Growth Direct System for the analysis of in-process samples in a biopharmaceutical environment. This paper describes the validation of the Growth Direct as an automation of the incubation and reading of the compendial plate method.
Four steps to validation
The paper describes the four steps to validating the Growth Direct System.
1. Installation/Operational Qualification (IOQ)
This step concentrates on the validation of the system’s hardware and software components to confirm that they are all functioning according to the design specification. This includes the calibration and temperature mapping of the incubators.
2. Performance Qualification (PQ)
The second step helps validate the microbiological method in accordance with those requirements of USP 1223 that apply to the Growth Direct technology.
This step also includes system count accuracy. The results for the enumeration accuracy performed during PQ for USP and environmental organisms are shown in Figure 1. A very good correlation is seen between the manual and system count for the test organisms. Data verify the enumeration accuracy of Growth Direct for organisms relevant to the site micro flora testing.
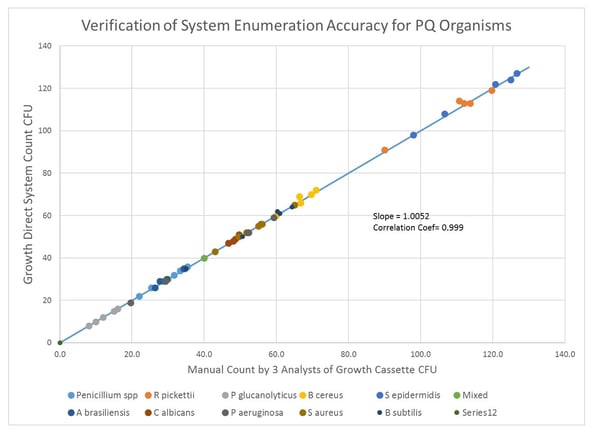
Fig 1 Correlation of system CFU counts for USP and EM microorganisms compared to the mean CFU count of 3 analysts on the same cassette.
3. Time to Result Qualification (TTRQ)
Following confirmation of the efficacy of the performance of the instrument, the time required to confirm a negative result is defined. A TTRQ is carried out.
Test samples of the organisms of interest from standard USP and in-house slow-growing environmental organisms are prepared and run on the system at 30-35°C. The colony detection profiles from the Growth Direct System are downloaded and analyzed. The TTR is taken as the point at which the recovery of the slowest growing organisms is acceptable. For the test samples in the study, the TTR was determined to be 36 hours, as shown in Figure 2.
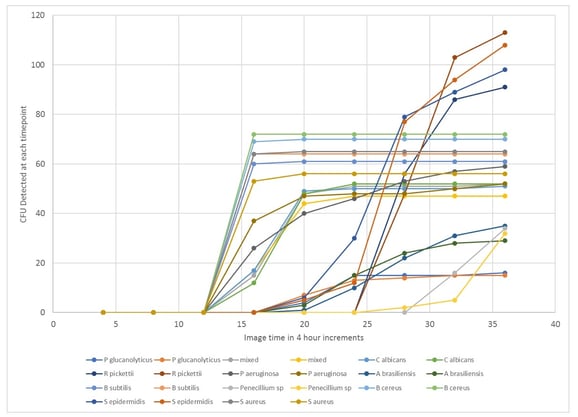
Fig 2 Estimation of Time to Results (TTR) from test samples run on the Growth Direct System. Each line represents the colony detection curve for each individual test sample.
4. Method suitability
The final phase of method suitability for the product evaluates interference, either to the growth of the organisms or interference of the product on the detection system of the Growth Direct technology.
Conclusion
Validation of the Growth Direct System to Perform Pharmaceutical In-process Bioburden Analysis describes the validation of the Growth Direct System as an automated colony counter applied to pharmaceutical in-process bioburden testing. The paper demonstrates that the Growth Direct System uses a proven strategy with a simplified verification approach following the process defined in the USP <1223> to show acceptability for in-process product testing.
To read Validation of the Growth Direct System to Perform Pharmaceutical In-process Bioburden Analysis, click here.
Check out our upcoming blogs for additional information on how to validate the Growth Direct using the simplified method of an automated colony counter.