blog
April 15, 2024 | Source: Rapid Micro Biosystems, Inc.
The Barriers to RMM Adoption
POSTED BY Rapid Micro Biosystems | 6 minute read
April 15, 2024 | Source: Rapid Micro Biosystems, Inc.
POSTED BY Rapid Micro Biosystems | 6 minute read
Between August and October 2023, we surveyed numerous experts within the pharmaceutical and biopharmaceutical sectors, gathering information and insight on the market requirements for RMMs. The survey encompassed participants from both the EU and the US, representing a mix of senior and junior employees across various roles, facility types, and categories of therapeutics.
The survey provided valuable insights, with 93% of participants expressing an interest in rapid sterility testing. Despite this, our research indicated a relatively low adoption rate among survey participants, with only 28% currently utilizing rapid sterility testing methods. This discrepancy between interest and adoption rates suggests multiple barriers are hindering the widespread implementation of Rapid Microbial Methods (RMMs) within the pharmaceutical and biopharmaceutical sectors.
In this blog, we discuss the barriers to adopting RMMs, and how the Growth Direct® system addresses these challenges.
Dive into expert insights on the key benefits of RMM
Despite the overwhelming interest in rapid sterility testing, significant barriers hinder widespread adoption. Addressing these obstacles is crucial to unlocking the full potential of RMMs and realizing their transformative impact on pharmaceutical manufacturing processes.
When we asked the 72% of respondents why they hadn’t adopted rapid sterility testing, we received a variety of responses. The three top concerns raised were the cost of instruments and tests, validation process, and training requirements (Figure 1).
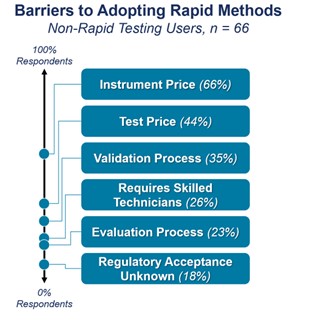
Figure 1. The main concerns from participants when considering implementing RMMs.
Price
There’s no denying that the adoption of RMMs in pharmaceutical manufacturing can involve significant costs, including those associated with the purchase, installation, qualification, and implementation. However, the benefits of RMMs — real-time results, automated workflow, enhanced accuracy, higher throughput, and superior data handling — contribute to substantial cost savings over time, ultimately yielding a positive ROI.
These potential savings include:
So, while the upfront investment in RMMs may seem daunting, the long-term benefits and potential cost savings offered by these technologies make them a strategic and financially prudent investment. Especially for pharmaceutical companies aiming to enhance their manufacturing processes and remain competitive in the industry.
Validation
The validation process for rapid platforms often raises concerns among experts due to its perceived complexity compared to traditional methods. However, regulatory agencies are accepting of RMMs and actively encourage their adoption.
Regulatory guidance, such as the PDA Technical Report #33, United States Pharmacopeia (USP) <1223>, and the European Pharmacopoeia (EP) chapter 5.1.6 offer direction on validation strategies, alleviating many of the concerns about the validation process.
Contact us for quick and easy support during your validation process
Training
After the initial implementation of a new RMM, staff will need time to adjust to the new workflow. However, following a training and transition period, they will quickly benefit from an automated workflow, streamlining operations and reducing manual labor.
The learning curve for the Growth Direct® System is particularly straightforward, as much of the protocol aligns with traditional methods commonly employed in the industry. Once technicians become proficient with our software, they can delegate the majority of the workflow tasks to the machine, freeing up valuable time and resources for other critical activities!
Request a demonstration to see how the Growth Direct® System could benefit your facility
Developments like advanced therapy medicinal products (ATMPs) are rapidly changing the pharmaceutical industry. Many ATMPs have short shelf lives, benefitting from rapid methods to ensure timely administration of safe products to patients. Consequently, regulatory policies in both the US and the EU are evolving to accommodate the unique requirements of short shelf-life products and the need for rapid testing.
At Rapid Micro Biosystems (RMB), we understand the critical importance of timely and accurate sterility testing in pharmaceutical manufacturing. Our Growth Direct® System is designed to meet these challenges head-on, offering unparalleled efficiency, reliability, and ease of use. By using advanced technology and our deep understanding of industry demands, we empower pharmaceutical manufacturers to overcome obstacles and navigate regulatory complexities with confidence, ultimately ensuring the delivery of safe and effective therapies to patients worldwide.