blog
April 11, 2024 | Source: Rapid Micro Biosystems, Inc.
The Benefits of Rapid Sterility Testing
POSTED BY Rapid Micro Biosystems | 9 minute read
April 11, 2024 | Source: Rapid Micro Biosystems, Inc.
POSTED BY Rapid Micro Biosystems | 9 minute read
Sterility testing is a core part of pharmaceutical manufacturing, ensuring that products remain free from harmful microorganisms. However, it is a time- and resource-intensive process that needs to be carried out under aseptic conditions by specialized staff following detailed protocols.
Traditional methods, such as membrane filtration and direct inoculation, can take weeks to yield results, slowing down product releases and investigations, should one be required. As a result, pharmaceutical manufacturers are increasingly investing in advanced sterility testing methods and technologies to increase efficiency while maintaining product safety and compliance.
One of the most significant advancements in pharmaceutical sterility testing is the adoption of Rapid Microbiological Methods (RMM). These innovative techniques have revolutionized the testing process, significantly reducing testing times, improving accuracy, and reducing the need for human intervention.
This blog explores our research on the market demand for RMMs, highlighting some key takeaways.
Discover how the Growth Direct® System can optimize your sterility testing
Between August and October 2023, we surveyed numerous experts within the pharmaceutical and biopharmaceutical sectors, gathering information and insight on the market requirements for RMMs. The survey encompassed participants from both the EU and the US, representing a mix of senior and junior employees across various roles, facility types, and categories of therapeutics.
Here's what we found.
An overwhelming majority (93%) of respondents expressed keen interest in rapid sterility testing. When asked to give their perceived advantages of rapid testing, the most frequently cited benefit was a ‘faster batch release’ (Figure 1). One expert emphasized that “sterility testing is one of the slowest tests in the whole process. Getting time back for products with a short shelf life makes a huge difference.” Faster batch release also minimizes warehouse storage time for non-cGMP products, which can lead to significant savings.
These survey findings correspond with the projected market growth rate of 8.20%, anticipated from 2023 to 2032, driven by a flourishing biotechnology industry and increasing concerns regarding the safety of biological products [1].
When participants were asked about the benefits of RMMs, the responses could be categorized into 4 main benefits: speed, operational efficiency, risk reduction, and enhanced data integrity. On further analysis, these benefits were subdivided into specific features, revealing the top five perceived advantages of RMM (Figure1):
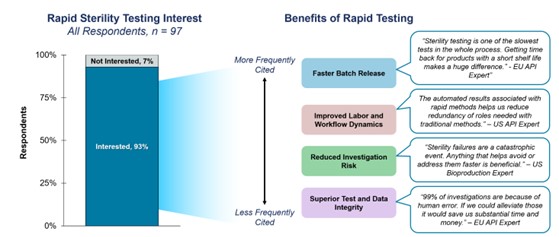
Figure 1. Bar chart showing the level of interest in rapid sterility testing, with the most cited benefits from participants.
An overview of the various benefits of RMMs and their level of importance for scientists is provided in Figure 2.
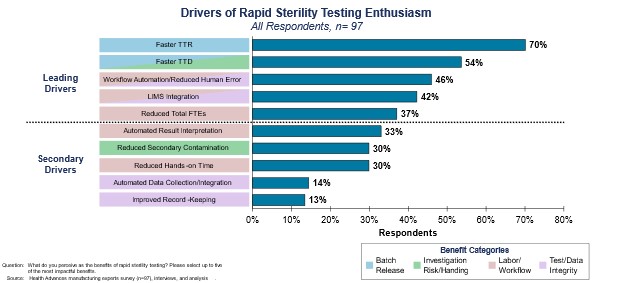
Figure 2. The most cited drivers for interest in rapid sterility tests.
Clearly, RMMs have a transformative impact on sterility testing processes, offering pharmaceutical manufacturers a comprehensive solution to enhance efficiency, accuracy, and regulatory compliance. However, despite the evident interest from scientists, and an understanding of the benefits, our research indicated a relatively low adoption rate of RMMs among survey participants (Figure 3).
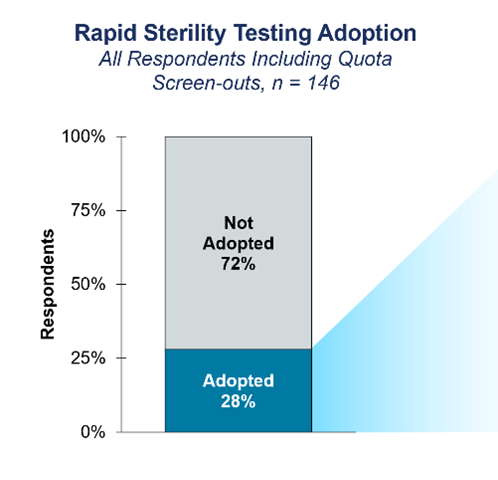
Figure 3. Bar chart showing the level of RMM adoption among participants.
Why could this be? Explore the potential barriers to RMM adoption in our next blog
Based on the insights gleaned from our survey participants, we have compiled a comprehensive outline detailing the essential features that an ideal RMM should have (Figure 4).
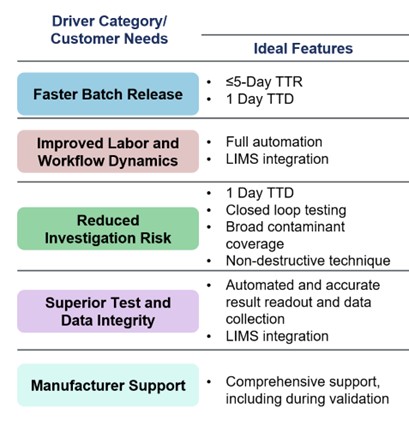
Figure 4. The ideal properties of an RMM, according to our participants
The participants were then given a "blinded" Target Product Profile (TPP) of the Growth Direct® System, showcasing its key product capabilities (Figure 5). These include:
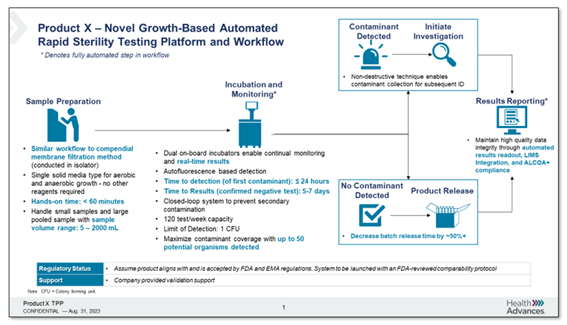
Figure 5. A "blinded" Target Product Profile (TPP) of the Growth Direct® System
We have listened to the experts, and found that the Growth Direct® System meets their essential needs. Overall, participants responded very positively to this product profile, with nearly 70% expressing an interest towards adopting the described technology.
This level of interest is significant, especially when considering that the Growth Direct® System can detect a contamination in as little as 1-3 days. This suggests a strong potential for widespread adoption and underscores the system's capability to meet the pressing demands of rapid microbial testing in pharmaceutical manufacturing.
Partnering for progress
As the pharmaceutical industry expands to encompass a vast array of products, from protein therapies to cell and gene-based therapeutics, manufacturing processes are becoming more complex. Ensuring the quality, safety, and efficacy of these biotherapeutics requires a multifaceted approach, integrating advanced technologies and stringent quality control measures.
Rapid Micro Biosystems (RMB) is at the forefront of this paradigm shift, demonstrating a deep understanding of market demands and a commitment to delivering cutting-edge solutions. By partnering with RMB, stakeholders gain access to not only advanced technology but also a wealth of expertise and support. Together, we can navigate the complexities of regulatory compliance, enhance operational efficiency, and pave the way for safer and more efficient drug development processes.
Request a demonstration today to see how the Growth Direct® System can benefit your facility.
References
1. Market Research Future. (2024). Rapid Sterility Testing Market Research, Size, Share, Industry Analysis By 2032. Retrieved 20 March 2024, from https://www.marketresearchfuture.com/reports/rapid-sterility-testing-market-6621