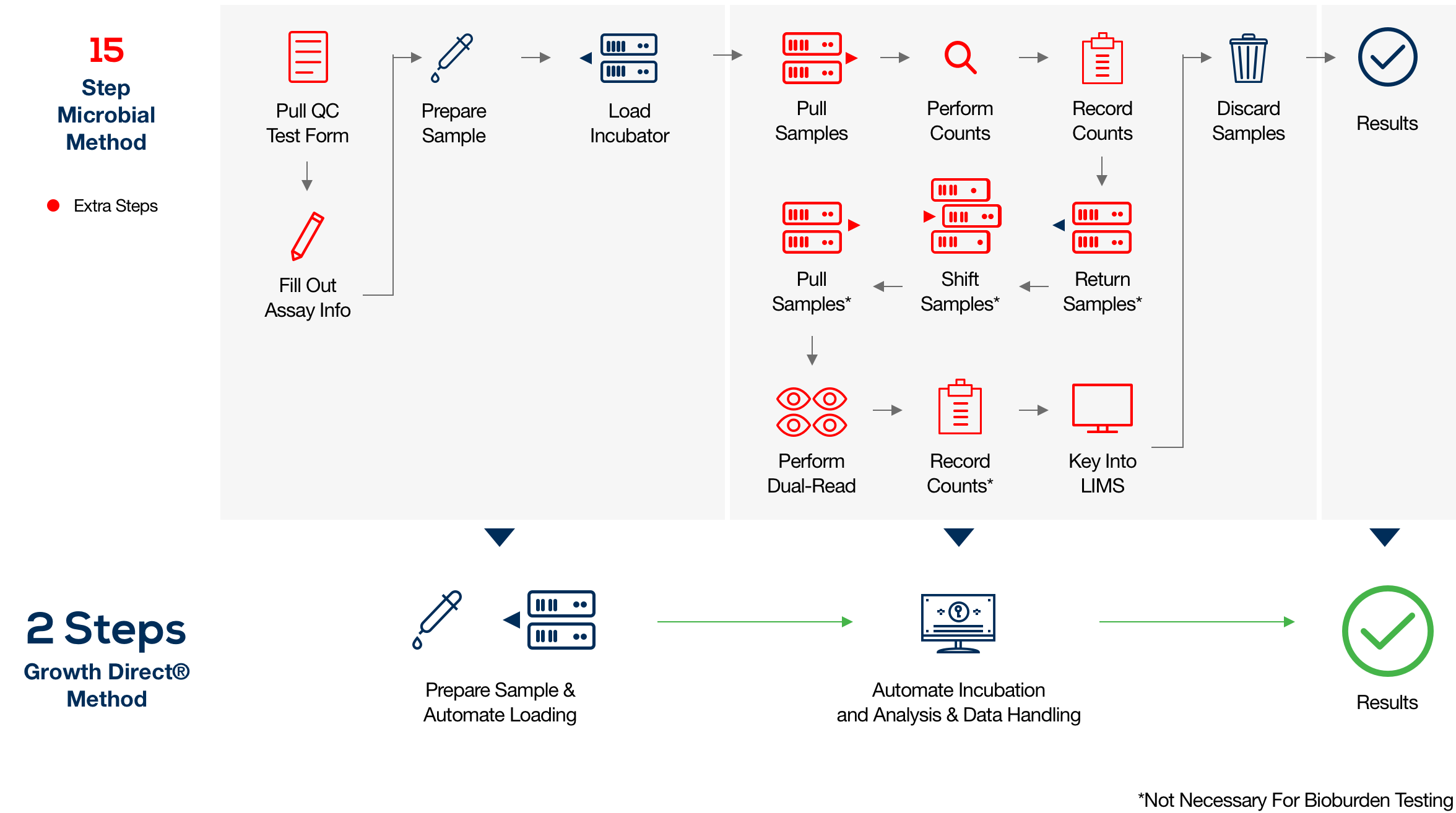
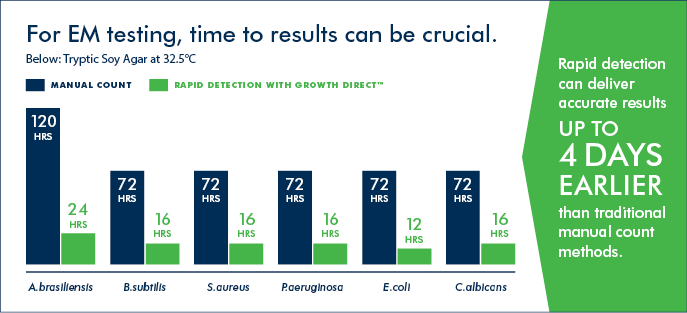
The Growth Direct® System is validated through a risk-based, regulatory-aligned approach tailored to environmental monitoring in GMP-compliant facilities. Most customers complete validation in approximately four months, supported by expert guidance and documentation.
Proven validation experience
Validated at leading pharmaceutical and biotech sites worldwide, Growth Direct® supports both standard qualification packages and site-specific PQ protocols.
Complete validation lifecycle
Our validation support includes:
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
- 21 CFR Part 11 compliance
- Method Qualification / Suitability (MQ/MS)
- Bi-directional LIMS integration
Expert-led, high-touch support
Validation begins prior to system purchase, with early consultation from a dedicated expert. Our team provides direct support throughout implementation—without triage delays.
Seamless LIMS and data integration
The system’s universal communication platform enables reliable .csv exports and integration with LIMS, trending databases, and quality systems.
.jpg)





.png?width=1200&height=729&name=Compendial%20Methods%20(2).png)